Abstract
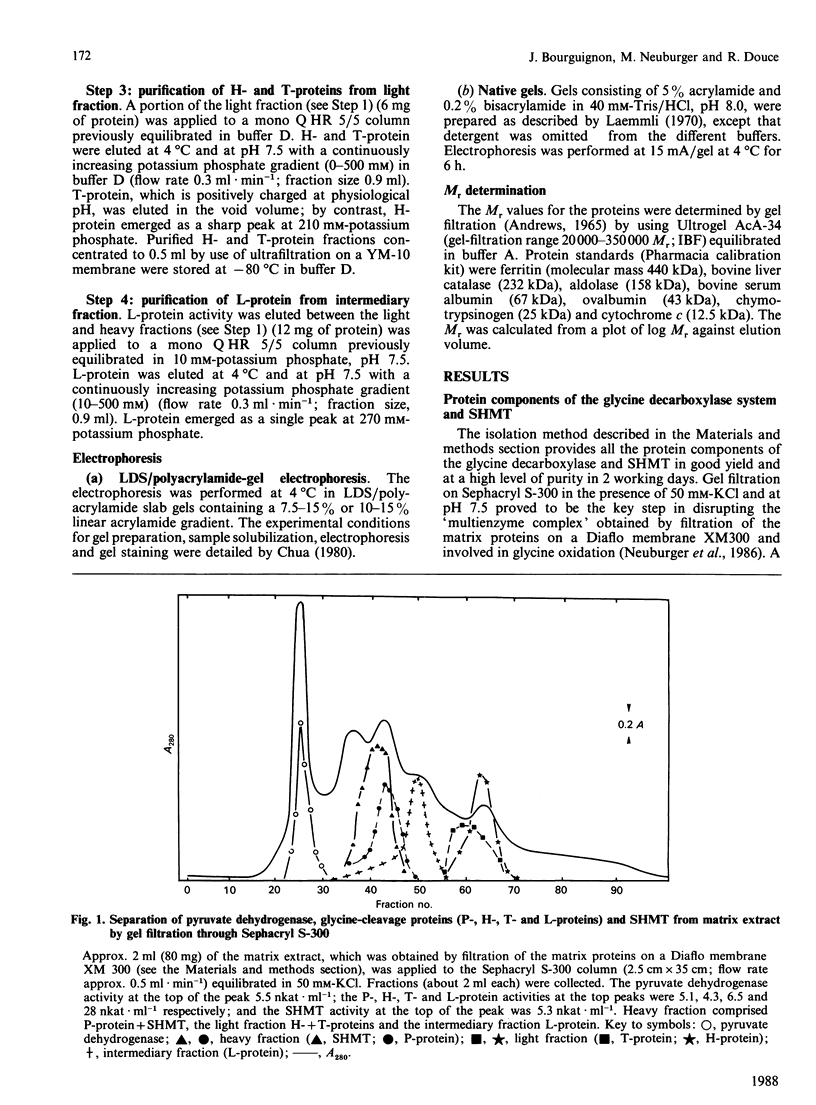
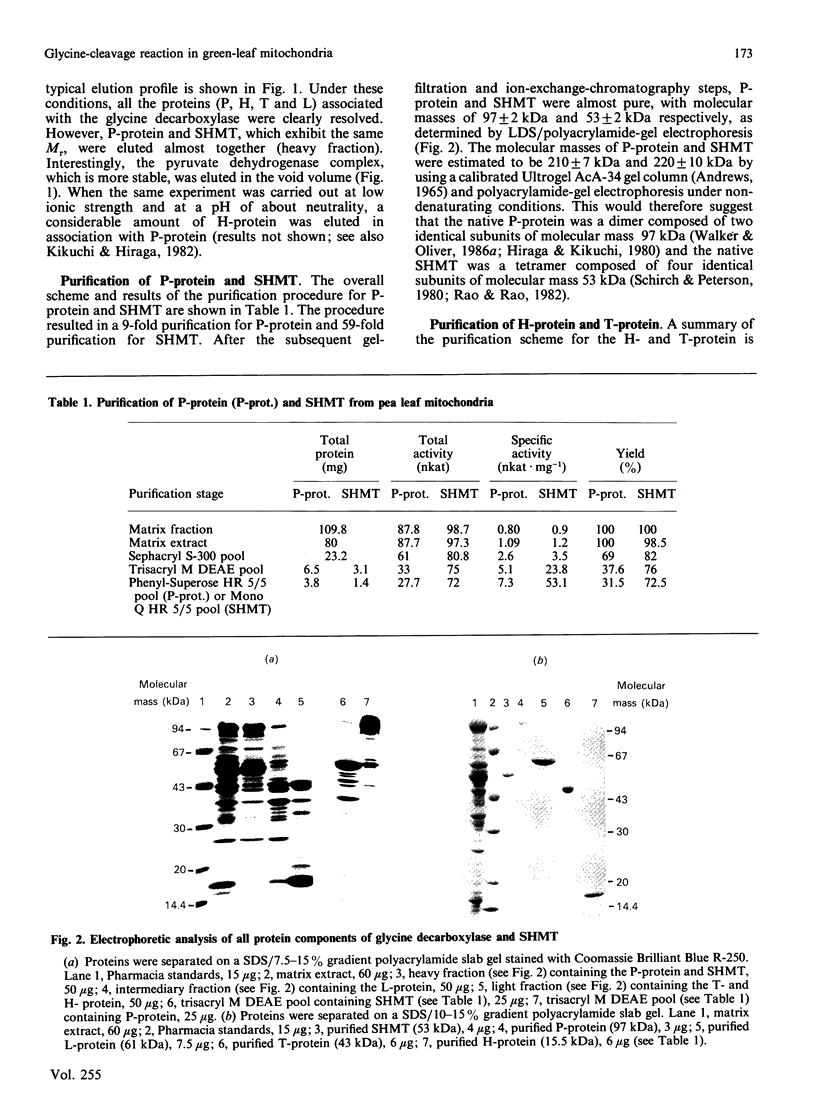
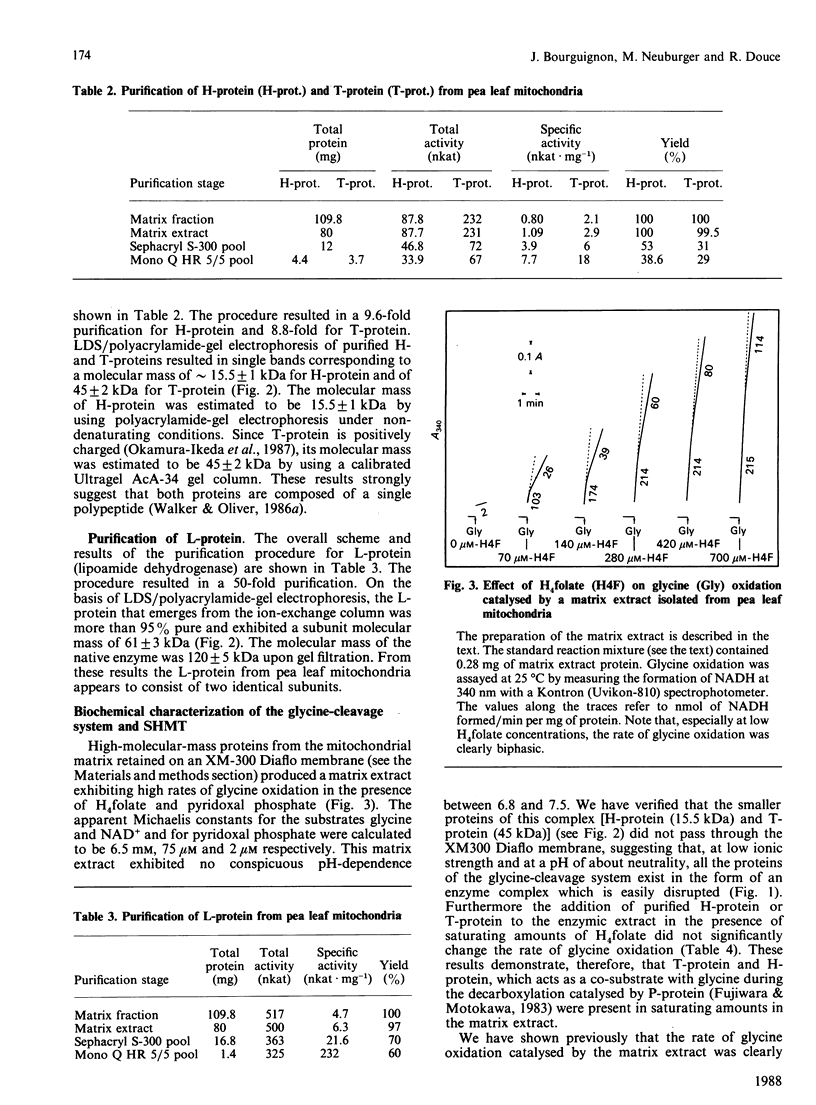
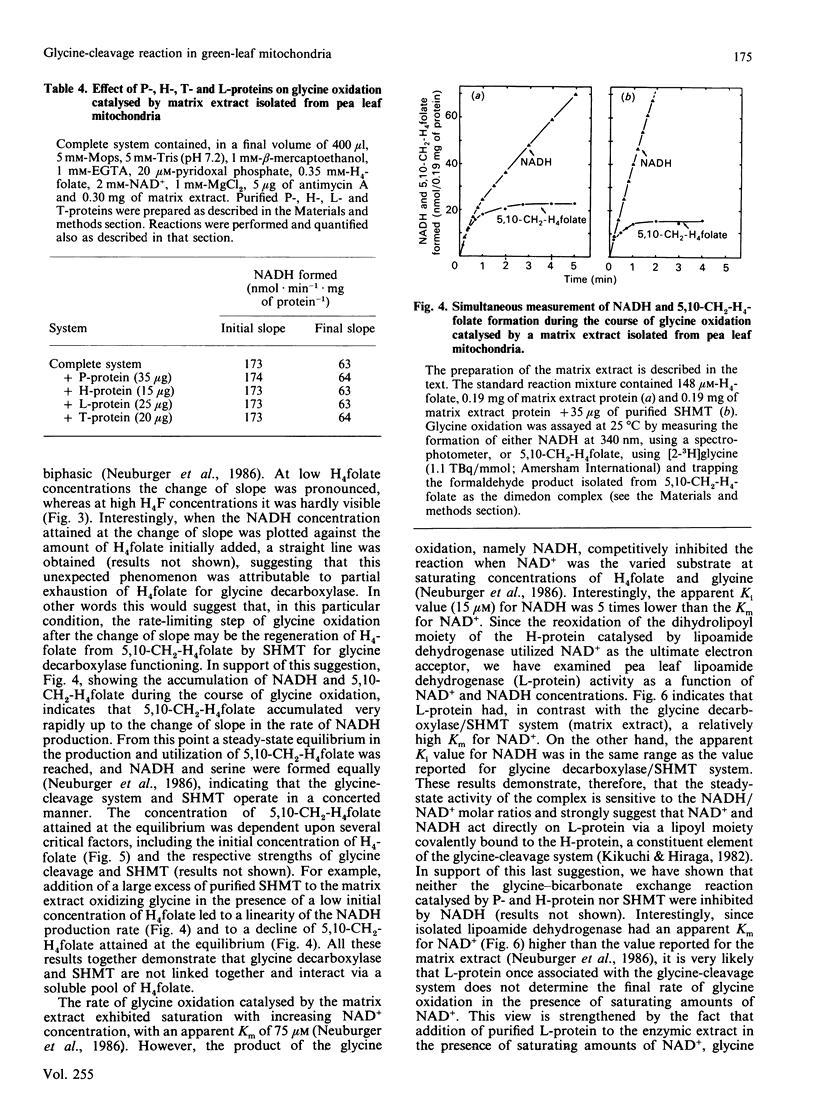
High-molecular-mass proteins from pea (Pisum sativum) mitochondrial matrix retained on an XM-300 Diaflo membrane ('matrix extract') exhibited high rates of glycine oxidation in the presence of NAD+ and tetrahydropteroyl-L-glutamic acid (H4 folate) as long as the medium exhibited a low ionic strength. Serine hydroxymethyltransferase (SHMT) (4 x 53 kDa) and the four proteins of the glycine-cleavage system, including a pyridoxal phosphate-containing enzyme ('P-protein'; 2 x 97 kDa), a carrier protein containing covalently bound lipoic acid ('H-protein'; 15.5 kDa), a protein exhibiting lipoamide dehydrogenase activity ('L-protein'; 2 x 61 kDa) and an H4 folate-dependent enzyme ('T-protein'; 45 kDa) have been purified to apparent homogeneity from the matrix extract by using gel filtration, ion-exchange and phenyl-Superose fast protein liquid chromatography. Gel filtration on Sephacryl S-300 in the presence of 50 mM-KCl proved to be the key step in disrupting this complex. During the course of glycine oxidation catalysed by the matrix extract a steady-state equilibrium in the production and utilization of 5,10-methylene-H4 folate was reached, suggesting that glycine cleavage and SHMT are linked together via a soluble pool of H4 folate. The rate of glycine oxidation catalysed by the matrix extract was sensitive to the NADH/NAD+ molar ratios, because NADH competitively inhibited the reaction catalysed by lipoamide dehydrogenase.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carothers D. J., Raefsky-Estrin C., Pons G., Patel M. S. Rat liver mitochondria contain two immunologically distinct dihydrolipoamide dehydrogenases. Arch Biochem Biophys. 1987 Aug 1;256(2):597–605. doi: 10.1016/0003-9861(87)90617-5. [DOI] [PubMed] [Google Scholar]
- Douce R., Bonner W. D., Jr Oxalacetate control of Krebs cycle oxidations in purified plant mitochondria. Biochem Biophys Res Commun. 1972 May 12;47(3):619–624. doi: 10.1016/0006-291x(72)90923-0. [DOI] [PubMed] [Google Scholar]
- Douce R., Christensen E. L., Bonner W. D., Jr Preparation of intaintact plant mitochondria. Biochim Biophys Acta. 1972 Aug 17;275(2):148–160. doi: 10.1016/0005-2728(72)90035-7. [DOI] [PubMed] [Google Scholar]
- Fujiwara K., Motokawa Y. Mechanism of the glycine cleavage reaction. Steady state kinetic studies of the P-protein-catalyzed reaction. J Biol Chem. 1983 Jul 10;258(13):8156–8162. [PubMed] [Google Scholar]
- Hiraga K., Kikuchi G. The mitochondrial glycine cleavage system. Purification and properties of glycine decarboxylase from chicken liver mitochondria. J Biol Chem. 1980 Dec 25;255(24):11664–11670. [PubMed] [Google Scholar]
- Kikuchi G., Hiraga K. The mitochondrial glycine cleavage system. Unique features of the glycine decarboxylation. Mol Cell Biochem. 1982 Jun 25;45(3):137–149. doi: 10.1007/BF00230082. [DOI] [PubMed] [Google Scholar]
- Klein S. M., Sagers R. D. Glycine metabolism. 3. A flavin-linked dehydrogenase associated with the glycine cleavage system in Peptococcus glycinophilus. J Biol Chem. 1967 Jan 25;242(2):297–300. [PubMed] [Google Scholar]
- Klein S. M., Sagers R. D. Glycine metabolism. I. Properties of the system catalyzing the exchange of bicarbonate with the carboxyl group of glycine in Peptococcus glycinophilus. J Biol Chem. 1966 Jan 10;241(1):197–205. [PubMed] [Google Scholar]
- Kochi H., Kikuchi G. Mechanism of reversible glycine cleavage reaction in Arthrobacter globiformis. Function of lipoic acid in the cleavage and synthesis of blycine. Arch Biochem Biophys. 1976 Mar;173(1):71–81. doi: 10.1016/0003-9861(76)90236-8. [DOI] [PubMed] [Google Scholar]
- Kochi H., Kikuchi G. Mechanism of the reversible glycine cleavage reaction in Arthrobacter globiformis. I. Purification and function of protein components required for the reaction. J Biochem. 1974 May;75(5):1113–1127. doi: 10.1093/oxfordjournals.jbchem.a130483. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miernyk J. A., Randall D. D. Some kinetic and regulatory properties of the pea mitochondrial pyruvate dehydrogenase complex. Plant Physiol. 1987 Feb;83(2):306–310. doi: 10.1104/pp.83.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura-Ikeda K., Fujiwara K., Motokawa Y. Purification and characterization of chicken liver T-protein, a component of the glycine cleavage system. J Biol Chem. 1982 Jan 10;257(1):135–139. [PubMed] [Google Scholar]
- Rao D. N., Rao N. A. Purification and regulatory properties of mung bean (vigna radiata L.) serine hydroxymethyltransferase. Plant Physiol. 1982 Jan;69(1):11–18. doi: 10.1104/pp.69.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarojini G., Oliver D. J. Extraction and partial characterization of the glycine decarboxylase multienzyme complex from pea leaf mitochondria. Plant Physiol. 1983 May;72(1):194–199. doi: 10.1104/pp.72.1.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirch L., Peterson D. Purification and properties of mitochondrial serine hydroxymethyltransferase. J Biol Chem. 1980 Aug 25;255(16):7801–7806. [PubMed] [Google Scholar]
- Schirch L. Serine hydroxymethyltransferase. Adv Enzymol Relat Areas Mol Biol. 1982;53:83–112. doi: 10.1002/9780470122983.ch3. [DOI] [PubMed] [Google Scholar]
- Walker J. L., Oliver D. J. Glycine decarboxylase multienzyme complex. Purification and partial characterization from pea leaf mitochondria. J Biol Chem. 1986 Feb 15;261(5):2214–2221. [PubMed] [Google Scholar]
- Walker J. L., Oliver D. J. Light-induced increases in the glycine decarboxylase multienzyme complex from pea leaf mitochondria. Arch Biochem Biophys. 1986 Aug 1;248(2):626–638. doi: 10.1016/0003-9861(86)90517-5. [DOI] [PubMed] [Google Scholar]



