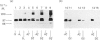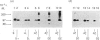Abstract
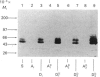
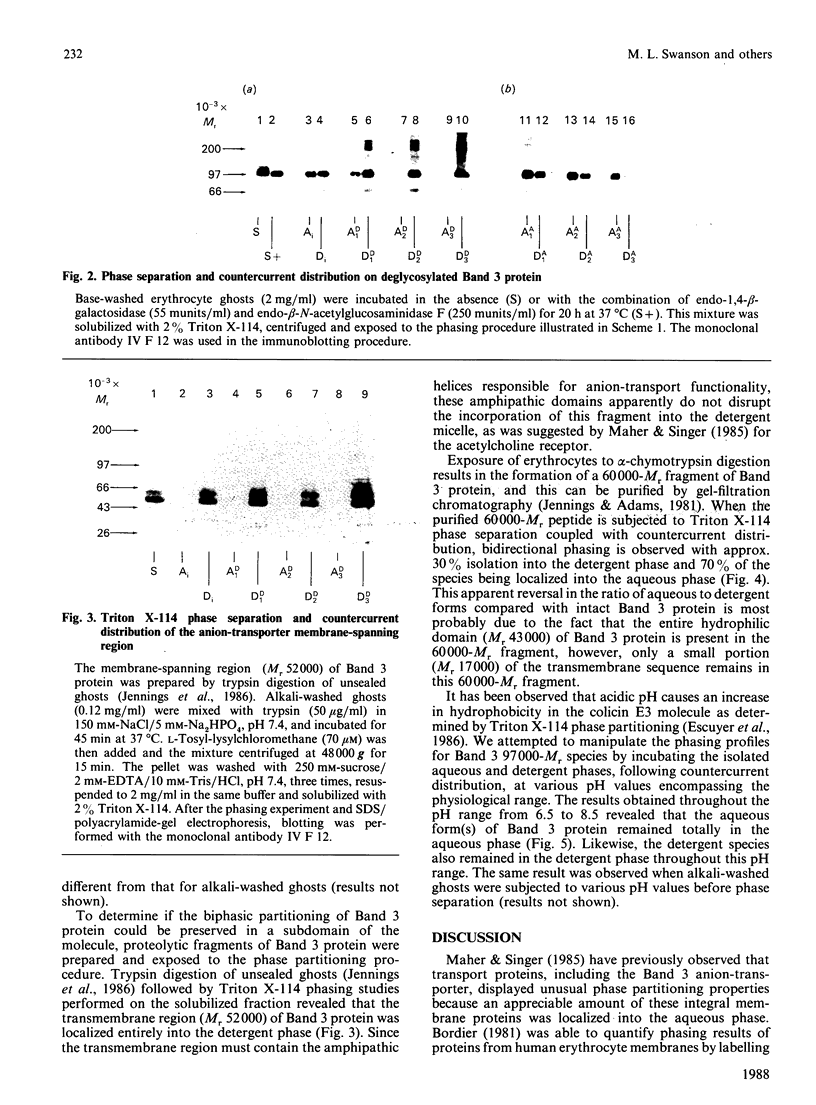
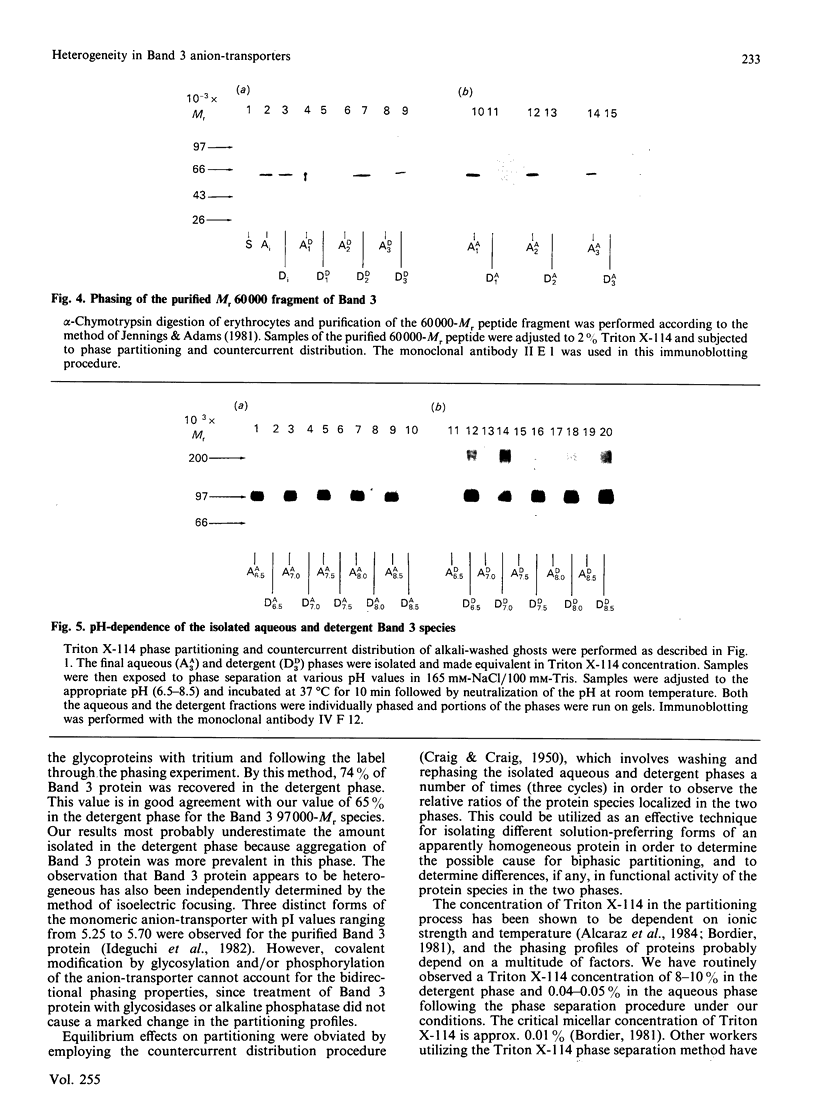
Triton X-114 phase partitioning used in conjunction with countercurrent distribution was utilized to examine the phasing properties of the human erythrocyte Band 3 anion-transport protein. Phase partitioning and countercurrent distribution of Band 3 protein followed by electrophoresis and immunoblotting revealed that Band 3 protein possesses biphasic properties with approx. 65% of the Band 3 97,000-Mr species being localized in the detergent phase and 35% isolated in the aqueous phase. The bidirectional phasing of the anion-transporter does not appear to be a result of glycosylation or phosphorylation, since treatment of alkali-washed ghosts with glycosidases or phosphatase respectively did not significantly alter the phasing profiles. Chymotrypsin treatment of erythrocytes followed by the purification of the 60,000-Mr fragment, and exposure of this fragment to phase separation and countercurrent distribution also revealed biphasic partitioning with 70% of the species being isolated in the aqueous phase and 30% in the detergent phase. These data demonstrate that the human erythrocyte Band 3 anion-transport protein is heterogenous by Triton X-114 phase partitioning and that this heterogeneity is preserved in the 60,000-Mr chymotryptic fragment of Band 3 protein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcaraz G., Kinet J. P., Kumar N., Wank S. A., Metzger H. Phase separation of the receptor for immunoglobulin E and its subunits in Triton X-114. J Biol Chem. 1984 Dec 10;259(23):14922–14927. [PubMed] [Google Scholar]
- Batteiger B., Newhall W. J., 5th, Jones R. B. The use of Tween 20 as a blocking agent in the immunological detection of proteins transferred to nitrocellulose membranes. J Immunol Methods. 1982 Dec 30;55(3):297–307. doi: 10.1016/0022-1759(82)90089-8. [DOI] [PubMed] [Google Scholar]
- Bennett V., Stenbuck P. J. Association between ankyrin and the cytoplasmic domain of band 3 isolated from the human erythrocyte membrane. J Biol Chem. 1980 Jul 10;255(13):6424–6432. [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Bouvier J., Etges R. J., Bordier C. Identification and purification of membrane and soluble forms of the major surface protein of Leishmania promastigotes. J Biol Chem. 1985 Dec 15;260(29):15504–15509. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brock C. J., Tanner M. J., Kempf C. The human erythrocyte anion-transport protein. Partial amino acid sequence, conformation and a possible molecular mechanism for anion exchange. Biochem J. 1983 Sep 1;213(3):577–586. doi: 10.1042/bj2130577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock C. J., Tanner M. J. The human erythrocyte anion-transport protein. Further amino acid sequence from the integral membrane domain homologous with the murine protein. Biochem J. 1986 May 1;235(3):899–901. doi: 10.1042/bj2350899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabantchik Z. I., Rothstein A. Membrane proteins related to anion permeability of human red blood cells. I. Localization of disulfonic stilbene binding sites in proteins involved in permeation. J Membr Biol. 1974;15(3):207–226. doi: 10.1007/BF01870088. [DOI] [PubMed] [Google Scholar]
- Carter-Su C., Pessin J. E., Mora R., Gitomer W., Czech M. P. Photoaffinity labeling of the human erythrocyte D-glucose transporter. J Biol Chem. 1982 May 25;257(10):5419–5425. [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Dekowski S. A., Rybicki A., Drickamer K. A tyrosine kinase associated with the red cell membrane phosphorylates band 3. J Biol Chem. 1983 Mar 10;258(5):2750–2753. [PubMed] [Google Scholar]
- Escuyer V., Boquet P., Perrin D., Montecucco C., Mock M. A pH-induced increase in hydrophobicity as a possible step in the penetration of colicin E3 through bacterial membranes. J Biol Chem. 1986 Aug 15;261(23):10891–10898. [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Findlay J. B. The receptor proteins for concanavalin A and Lens culinaris phytohemagglutinin in the membrane of the human erythrocyte. J Biol Chem. 1974 Jul 25;249(14):4398–4403. [PubMed] [Google Scholar]
- Fukuda M. N., Fukuda M., Hakomori S. Cell surface modification by endo-beta-galactosidase. Change of blood group activities and release of oligosaccharides from glycoproteins and glycosphingolipids of human erythrocytes. J Biol Chem. 1979 Jun 25;254(12):5458–5465. [PubMed] [Google Scholar]
- Generoso W. M., Cain K. T., Krishna M., Huff S. W. Genetic lesions induced by chemicals in spermatozoa and spermatids of mice are repaired in the egg. Proc Natl Acad Sci U S A. 1979 Jan;76(1):435–437. doi: 10.1073/pnas.76.1.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorga F. R., Lienhard G. E. Equilibria and kinetics of ligand binding to the human erythrocyte glucose transporter. Evidence for an alternating conformation model for transport. Biochemistry. 1981 Sep 1;20(18):5108–5113. doi: 10.1021/bi00521a003. [DOI] [PubMed] [Google Scholar]
- Hargreaves W. R., Giedd K. N., Verkleij A., Branton D. Reassociation of ankyrin with band 3 in erythrocyte membranes and in lipid vesicles. J Biol Chem. 1980 Dec 25;255(24):11965–11972. [PubMed] [Google Scholar]
- Ho M. K., Guidotti G. A membrane protein from human erythrocytes involved in anion exchange. J Biol Chem. 1975 Jan 25;250(2):675–683. [PubMed] [Google Scholar]
- Holm C., Fredrikson G., Belfrage P. Demonstration of the amphiphilic character of hormone-sensitive lipase by temperature-induced phase separation in Triton X-114 and charge-shift electrophoresis. J Biol Chem. 1986 Nov 25;261(33):15659–15661. [PubMed] [Google Scholar]
- Ideguchi H., Matsuyama H., Hamasaki N. Heterogeneity of human erythrocyte band 3 analyzed by two-dimensional gel electrophoresis. Eur J Biochem. 1982 Jul;125(3):665–671. doi: 10.1111/j.1432-1033.1982.tb06734.x. [DOI] [PubMed] [Google Scholar]
- Jennings M. L., Adams M. F. Modification by papain of the structure and function of band 3, the erythrocyte anion transport protein. Biochemistry. 1981 Dec 8;20(25):7118–7123. doi: 10.1021/bi00528a011. [DOI] [PubMed] [Google Scholar]
- Jennings M. L., Anderson M. P., Monaghan R. Monoclonal antibodies against human erythrocyte band 3 protein. Localization of proteolytic cleavage sites and stilbenedisulfonate-binding lysine residues. J Biol Chem. 1986 Jul 5;261(19):9002–9010. [PubMed] [Google Scholar]
- Kopito R. R., Lodish H. F. Primary structure and transmembrane orientation of the murine anion exchange protein. Nature. 1985 Jul 18;316(6025):234–238. doi: 10.1038/316234a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maher P. A., Singer S. J. Anomalous interaction of the acetylcholine receptor protein with the nonionic detergent Triton X-114. Proc Natl Acad Sci U S A. 1985 Feb;82(4):958–962. doi: 10.1073/pnas.82.4.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsas R., Stephenson S. L., Hryszko J., Kenny A. J., Turner A. J. The metabolism of neuropeptides. Phase separation of synaptic membrane preparations with Triton X-114 reveals the presence of aminopeptidase N. Biochem J. 1985 Oct 15;231(2):445–449. doi: 10.1042/bj2310445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawby W. J., Findlay J. B. Characterization and partial sequence of di-iodosulphophenyl isothiocyanate-binding peptide from human erythrocyte anion-transport protein. Biochem J. 1982 Sep 1;205(3):465–475. doi: 10.1042/bj2050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternack G. R., Anderson R. A., Leto T. L., Marchesi V. T. Interactions between protein 4.1 and band 3. An alternative binding site for an element of the membrane skeleton. J Biol Chem. 1985 Mar 25;260(6):3676–3683. [PubMed] [Google Scholar]
- Pryde J. G., Phillips J. H. Fractionation of membrane proteins by temperature-induced phase separation in Triton X-114. Application to subcellular fractions of the adrenal medulla. Biochem J. 1986 Jan 15;233(2):525–533. doi: 10.1042/bj2330525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner M. J., Boxer D. H. Separation and some properties of the major proteins of the human erythrocyte membrane. Biochem J. 1972 Sep;129(2):333–347. doi: 10.1042/bj1290333. [DOI] [PMC free article] [PubMed] [Google Scholar]