Abstract
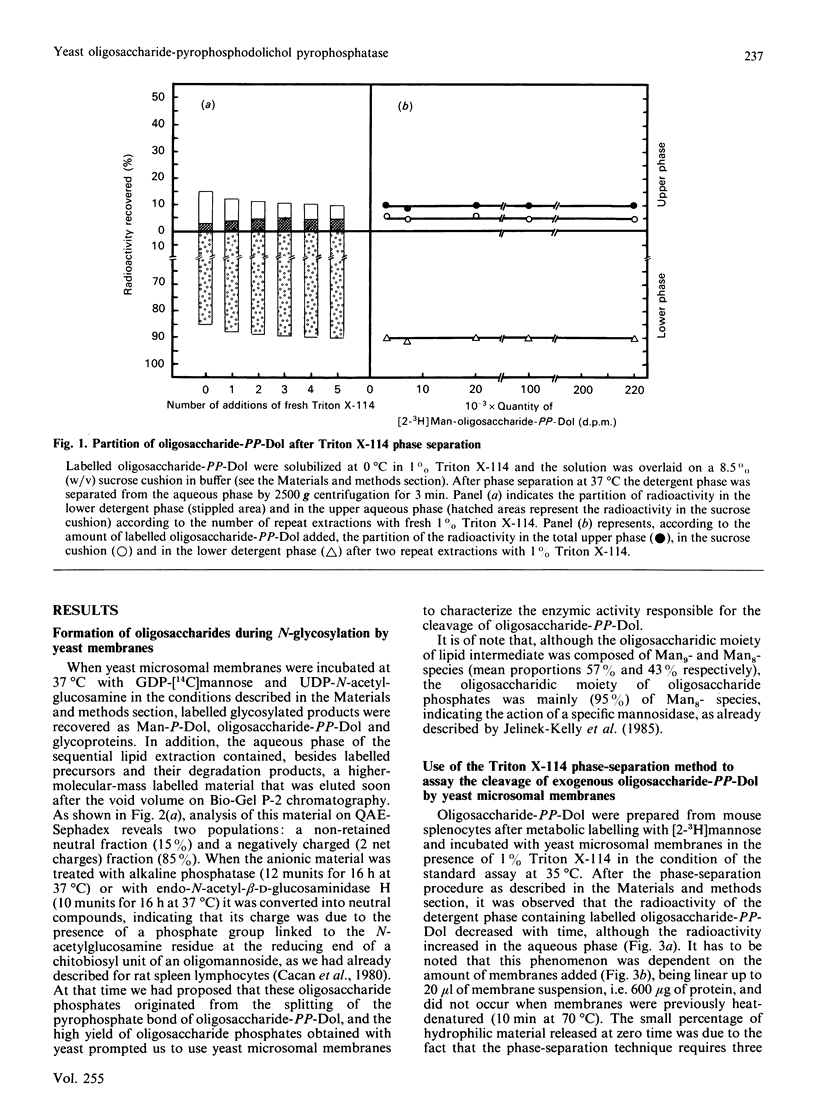
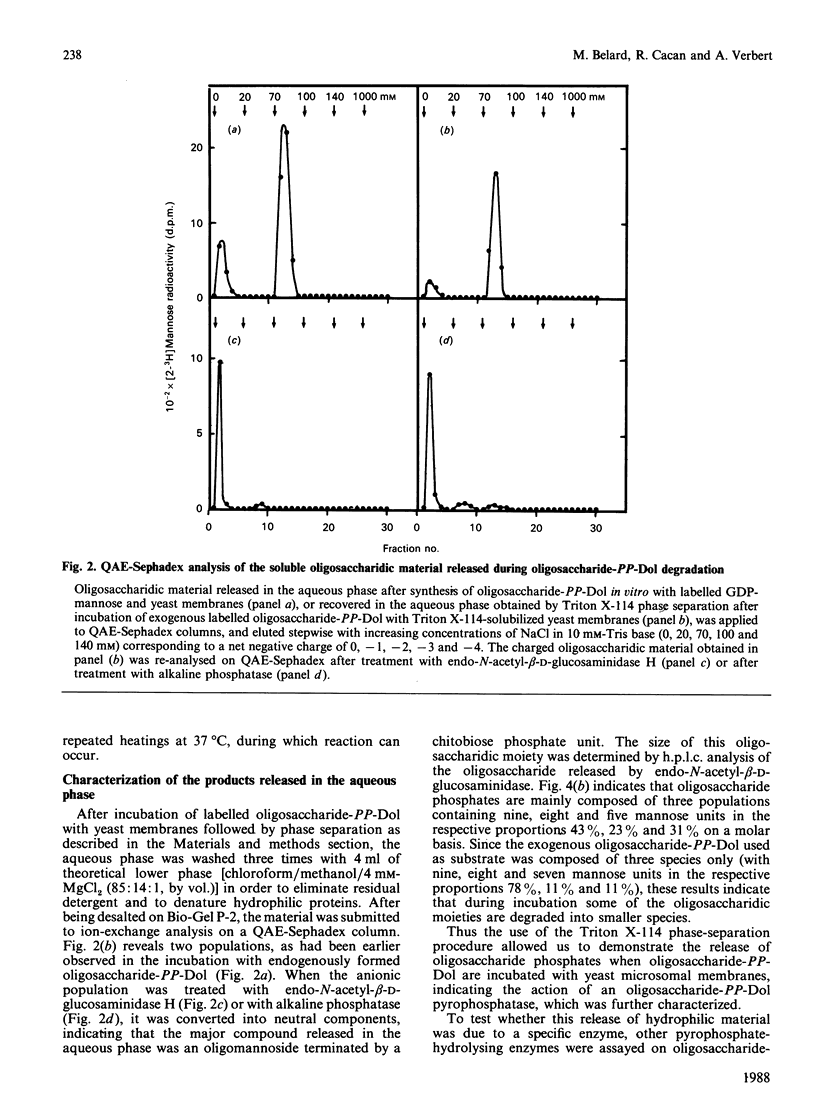
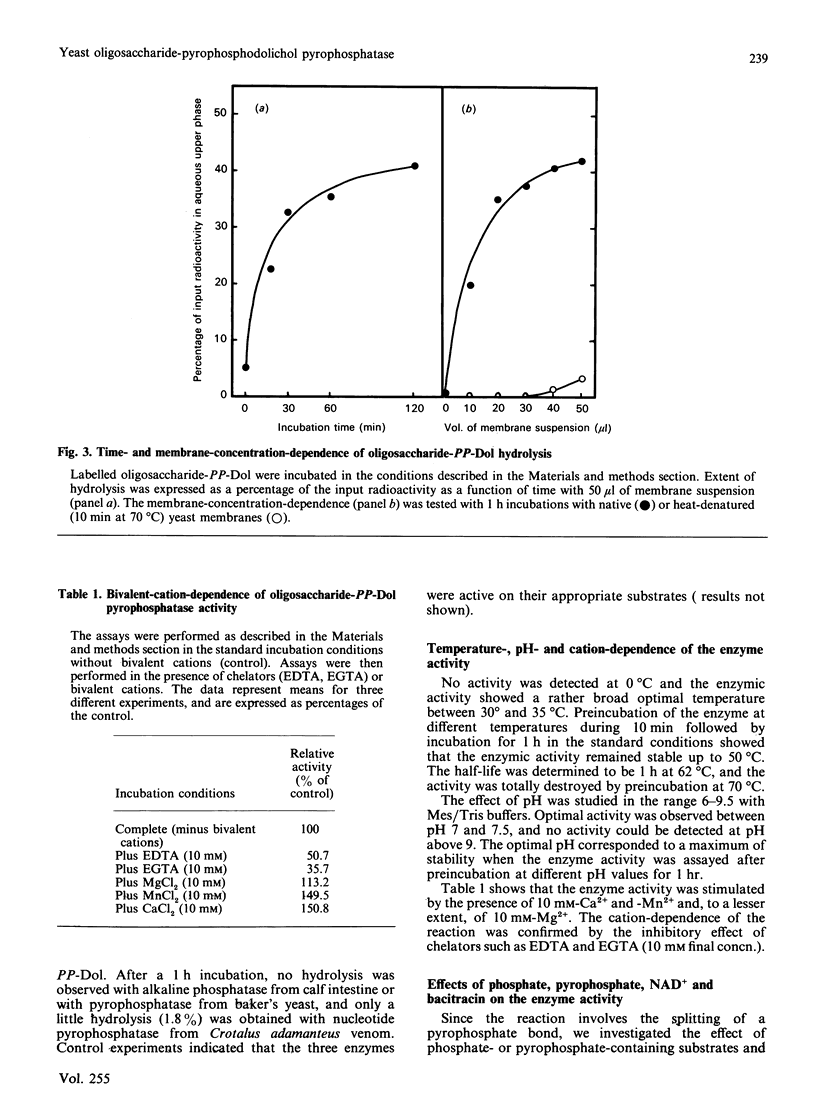
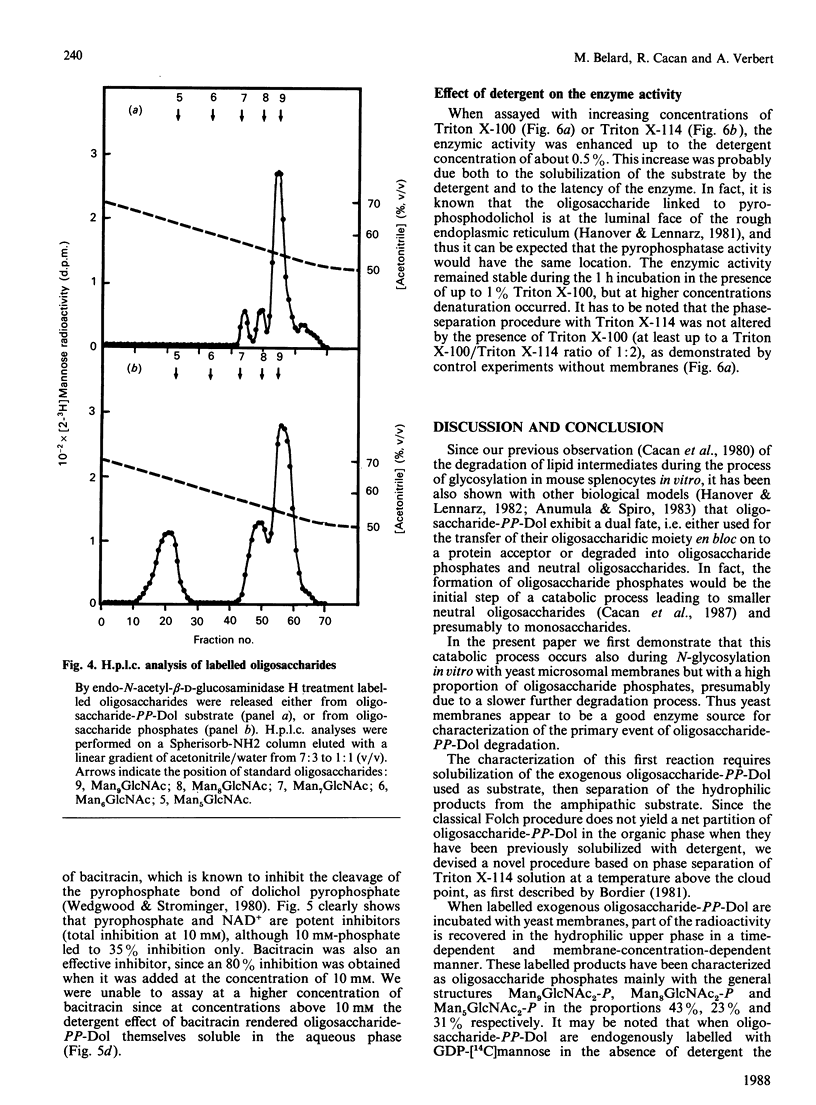
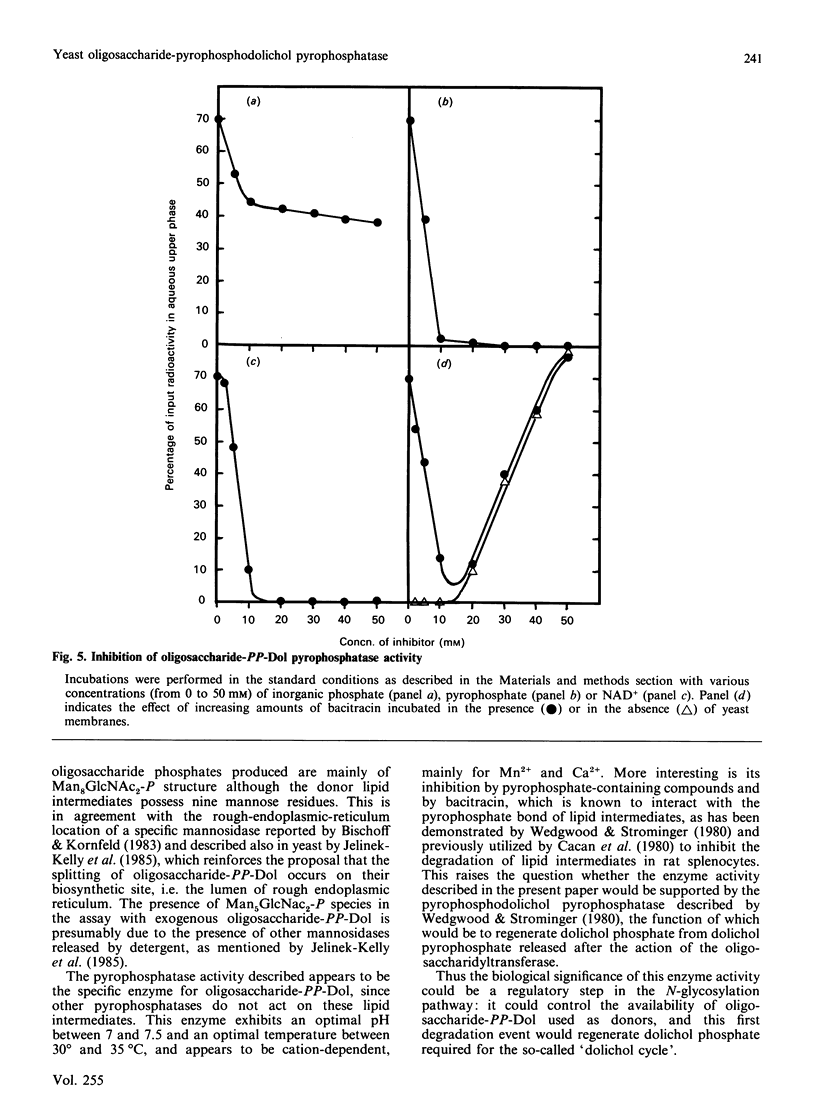
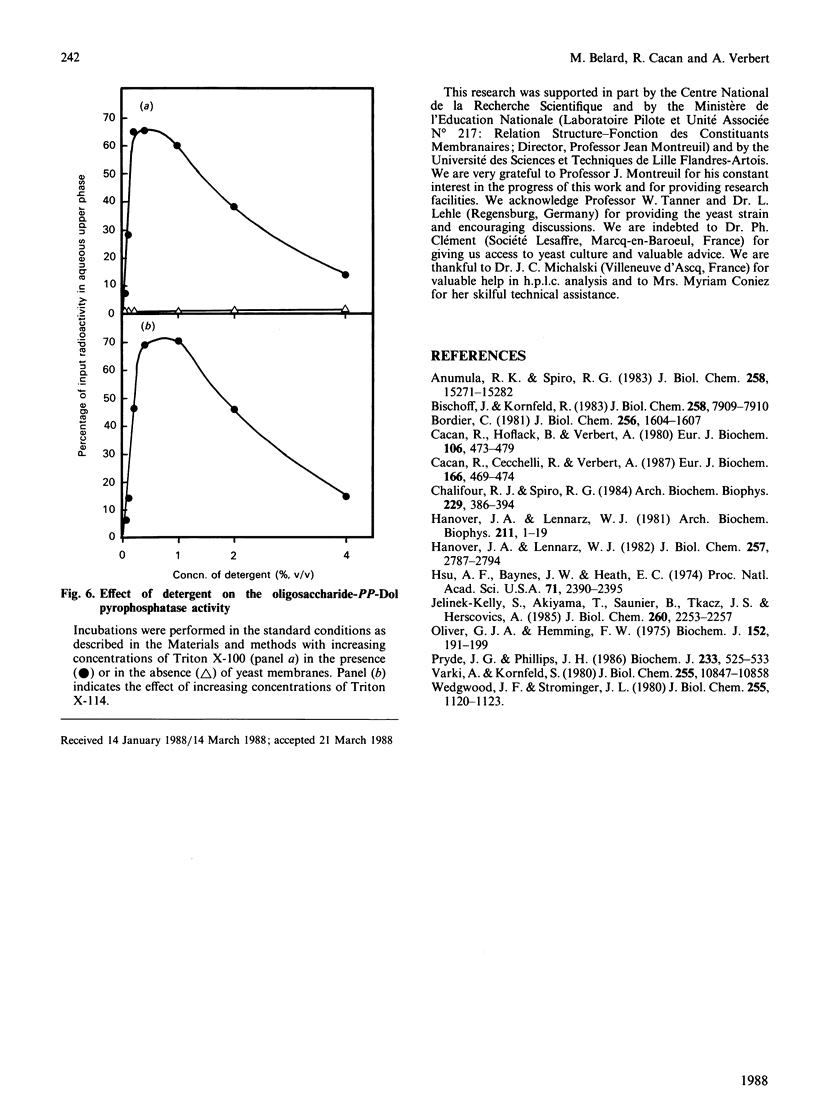
Use of Triton X-114 allowed us to develop a new method to separate hydrophilic oligosaccharidic material from hydrophobic oligosaccharide pyrophosphodolichols (oligosaccharide-PP-Dol). Taking advantage of this procedure we characterize, in yeast microsomal membranes, an enzymic activity that hydrolyses oligosaccharide-PP-Dol into oligosaccharidic material. H.p.l.c. analysis together with alkaline-phosphatase- and endo-N-acetyl-beta-D-glucosaminidase-susceptibility demonstrate that the oligosaccharidic released material is mainly composed of oligomannosides containing a chitobiose phosphate at the reducing end. The enzymic activity requires bivalent cations and is inhibited by pyrophosphate, NAD+ and bacitracin. As other, commercially available, pyrophosphatases have no action on lipid intermediates, the described pyrophosphatase activity appears to be the specific enzyme for oligosaccharide-PP-Dol. This enzymic splitting of the pyrophosphate bond might be the primary event in the catabolism of lipid intermediates.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anumula K. R., Spiro R. G. Release of glucose-containing polymannose oligosaccharides during glycoprotein biosynthesis. Studies with thyroid microsomal enzymes and slices. J Biol Chem. 1983 Dec 25;258(24):15274–15282. [PubMed] [Google Scholar]
- Bischoff J., Kornfeld R. Evidence for an alpha-mannosidase in endoplasmic reticulum of rat liver. J Biol Chem. 1983 Jul 10;258(13):7907–7910. [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Cacan R., Cecchelli R., Verbert A. Catabolic pathway of oligosaccharide-diphospho-dolichol. Study of the fate of the oligosaccharidic moiety in mouse splenocytes. Eur J Biochem. 1987 Jul 15;166(2):469–474. doi: 10.1111/j.1432-1033.1987.tb13539.x. [DOI] [PubMed] [Google Scholar]
- Cacan R., Hoflack B., Verbert A. Fate of oligosaccharide-lipid intermediates synthesized by resting rat-spleen lymphocytes. Eur J Biochem. 1980 May;106(2):473–479. doi: 10.1111/j.1432-1033.1980.tb04594.x. [DOI] [PubMed] [Google Scholar]
- Chalifour R. J., Spiro R. G. Cleavage of dolichyl pyrophosphoryl oligosaccharides by endo-beta-N-acetylglucosaminidase H: comparison of enzymatic and acid hydrolysis techniques for saccharide release. Arch Biochem Biophys. 1984 Feb 15;229(1):386–394. doi: 10.1016/0003-9861(84)90166-8. [DOI] [PubMed] [Google Scholar]
- Hanover J. A., Lennarz W. J. Transmembrane assembly of N-linked glycoproteins. Studies on the topology of saccharide synthesis. J Biol Chem. 1982 Mar 25;257(6):2787–2794. [PubMed] [Google Scholar]
- Hanover J. A., Lennarz W. J. Transmembrane assembly of membrane and secretory glycoproteins. Arch Biochem Biophys. 1981 Oct 1;211(1):1–19. doi: 10.1016/0003-9861(81)90423-9. [DOI] [PubMed] [Google Scholar]
- Hsu A. F., Baynes J. W., Heath E. C. The role of a dolichol-oligosaccharide as an intermediate in glycoprotein biosynthesis. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2391–2395. doi: 10.1073/pnas.71.6.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek-Kelly S., Akiyama T., Saunier B., Tkacz J. S., Herscovics A. Characterization of a specific alpha-mannosidase involved in oligosaccharide processing in Saccharomyces cerevisiae. J Biol Chem. 1985 Feb 25;260(4):2253–2257. [PubMed] [Google Scholar]
- Oliver G. J., Hemming F. W. The transfer of mannose to dolichol diphosphate oligosaccharides in pig liver endoplasmic reticulum. Biochem J. 1975 Nov;152(2):191–199. doi: 10.1042/bj1520191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryde J. G., Phillips J. H. Fractionation of membrane proteins by temperature-induced phase separation in Triton X-114. Application to subcellular fractions of the adrenal medulla. Biochem J. 1986 Jan 15;233(2):525–533. doi: 10.1042/bj2330525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A., Kornfeld S. Structural studies of phosphorylated high mannose-type oligosaccharides. J Biol Chem. 1980 Nov 25;255(22):10847–10858. [PubMed] [Google Scholar]
- Wedgwood J. F., Strominger J. L. Enzymatic activities in cultured human lymphocytes that dephosphorylate dolichyl pyrophosphate and dolichyl phosphate. J Biol Chem. 1980 Feb 10;255(3):1120–1123. [PubMed] [Google Scholar]


