Abstract
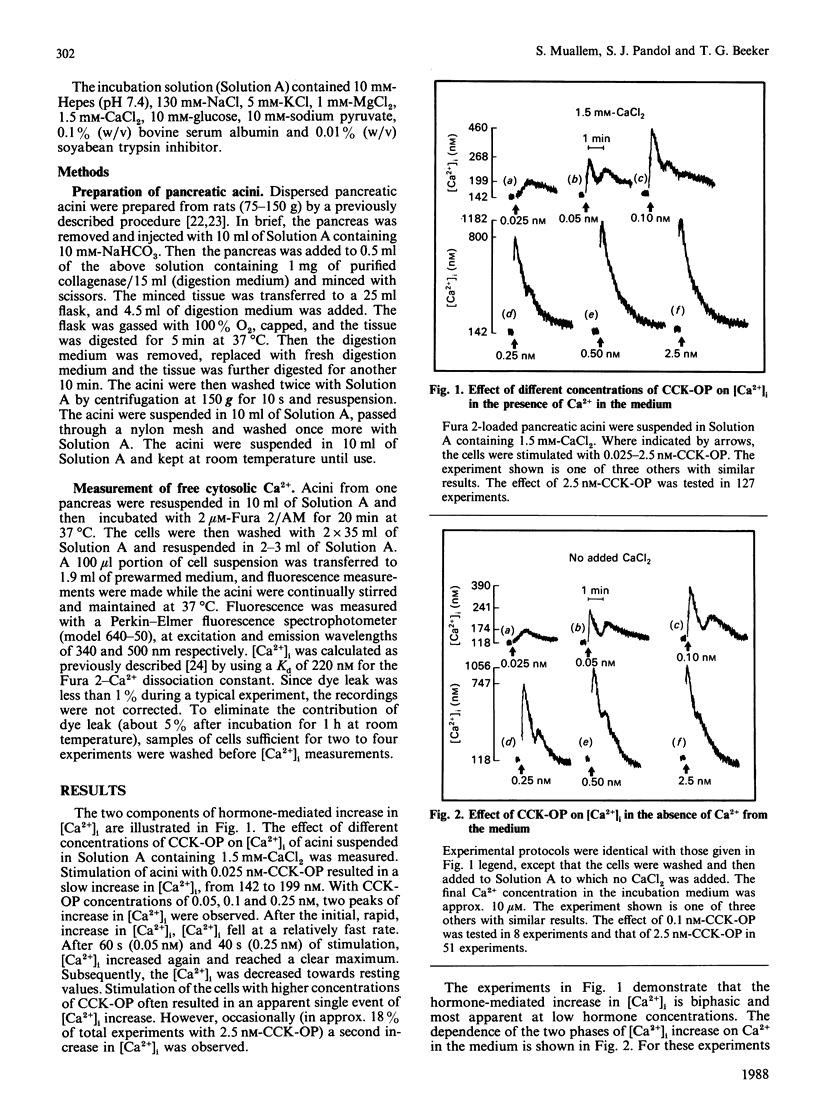
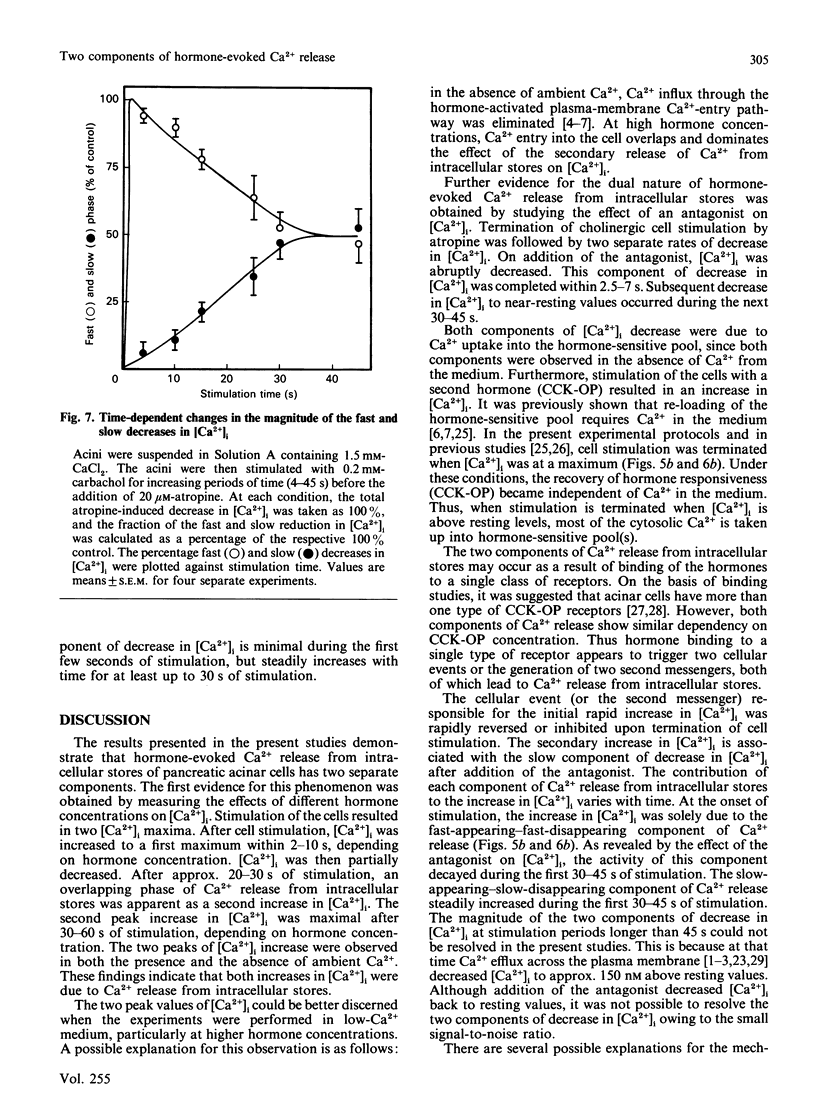
Dispersed pancreatic acini loaded with Fura 2 were used to study the effect of hormonal stimulation on [Ca2+]i (free cytosolic Ca2+ concentration). Stimulation of acini with cholecystokinin octapeptide or carbachol resulted in two components of increase in [Ca2+]i. The maximal increase in [Ca2+]i and the time to maximum for both components was dependent on hormone concentration. The first component reached a maximum after 2-10 s of stimulation, whereas the second component required 30-60 s of stimulation for maximal effect. Both components of the [Ca2+]i increase can be observed in the presence or absence of Ca2+ in the incubation medium. The two components of Ca2+ release from intracellular stores showed similar dependency on agonist concentration. Termination of cell stimulation with specific antagonist revealed two, kinetically separated, rates of decrease in [Ca2+]i. The initial decrease in [Ca2+]i, was completed within 2.5-7 s, whereas the secondary decrease in [Ca2+]i, back to resting values, required approx. 40 s. The magnitude of the antagonist-induced initial (rapid) and secondary (slow) decrease in [Ca2+]i was dependent on the duration of cell stimulation. Hence it appears that stimulation of pancreatic acinar cells with Ca2+-mobilizing hormones results in two, kinetically separated, components of Ca2+ release from intracellular stores.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bruzzone R., Halban P. A., Gjinovci A., Trimble E. R. A new, rapid, method for preparation of dispersed pancreatic acini. Biochem J. 1985 Mar 1;226(2):621–624. doi: 10.1042/bj2260621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon J. F., Hokin L. E. Inositol 1,2-cyclic 4,5-trisphosphate concentration relative to inositol 1,4,5-trisphosphate in pancreatic minilobules on stimulation with carbamylcholine in the absence of lithium. Possible role as a second messenger in long- but not short-term responses. J Biol Chem. 1987 Oct 15;262(29):13892–13895. [PubMed] [Google Scholar]
- Dormer R. L., Poulsen J. H., Licko V., Williams J. A. Calcium fluxes in isolated pancreatic acini: effects of secretagogues. Am J Physiol. 1981 Jan;240(1):G38–G49. doi: 10.1152/ajpgi.1981.240.1.G38. [DOI] [PubMed] [Google Scholar]
- Gardner J. D., Conlon T. P., Kleveman H. L., Adams T. D., Ondetti M. A. Action of cholecystokinin and cholinergic agents on calcium transport in isolated pancreatic acinar cells. J Clin Invest. 1975 Aug;56(2):366–375. doi: 10.1172/JCI108101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hokin L. E. Receptors and phosphoinositide-generated second messengers. Annu Rev Biochem. 1985;54:205–235. doi: 10.1146/annurev.bi.54.070185.001225. [DOI] [PubMed] [Google Scholar]
- Imboden J. B., Weiss A. The T-cell antigen receptor regulates sustained increases in cytoplasmic free Ca2+ through extracellular Ca2+ influx and ongoing intracellular Ca2+ mobilization. Biochem J. 1987 Nov 1;247(3):695–700. doi: 10.1042/bj2470695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Anggård E. E., Letcher A. J., Downes C. P. Metabolism of inositol 1,4,5-trisphosphate and inositol 1,3,4-trisphosphate in rat parotid glands. Biochem J. 1985 Jul 15;229(2):505–511. doi: 10.1042/bj2290505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Lander D. J., Berridge M. J. Specificity of inositol phosphate-stimulated Ca2+ mobilization from Swiss-mouse 3T3 cells. Biochem J. 1986 Nov 15;240(1):301–304. doi: 10.1042/bj2400301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii H., Connolly T. M., Bross T. E., Majerus P. W. Inositol cyclic triphosphate [inositol 1,2-(cyclic)-4,5-triphosphate] is formed upon thrombin stimulation of human platelets. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6397–6401. doi: 10.1073/pnas.83.17.6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. T., Lemp G. F., Gardner J. D. Interaction of cholecystokinin with specific membrane receptors on pancreatic acinar cells. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2079–2083. doi: 10.1073/pnas.77.4.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph S. K., Williams R. J. Subcellular localization and some properties of the enzymes hydrolysing inositol polyphosphates in rat liver. FEBS Lett. 1985 Jan 28;180(2):150–154. doi: 10.1016/0014-5793(85)81061-9. [DOI] [PubMed] [Google Scholar]
- Joseph S. K., Williamson J. R. Characteristics of inositol trisphosphate-mediated Ca2+ release from permeabilized hepatocytes. J Biol Chem. 1986 Nov 5;261(31):14658–14664. [PubMed] [Google Scholar]
- Matthews E. K., Petersen O. H., Williams J. A. Pancreatic acinar cells: acetylcholine-induced membrane depolarization, calcium efflux and amylase release. J Physiol. 1973 Nov;234(3):689–701. doi: 10.1113/jphysiol.1973.sp010367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muallem S., Beeker T. G., Fimmel C. J. Activation of the endoplasmic reticulum Ca2+ pump of pancreatic acini by Ca2+ mobilizing hormones. Biochem Biophys Res Commun. 1987 Nov 30;149(1):213–220. doi: 10.1016/0006-291x(87)91626-3. [DOI] [PubMed] [Google Scholar]
- Muallem S., Beeker T., Pandol S. J. Role of Na+/Ca2+ exchange and the plasma membrane Ca2+ pump in hormone-mediated Ca2+ efflux from pancreatic acini. J Membr Biol. 1988 May;102(2):153–162. doi: 10.1007/BF01870453. [DOI] [PubMed] [Google Scholar]
- Muallem S., Fimmel C. J., Pandol S. J., Sachs G. Regulation of free cytosolic Ca2+ in the peptic and parietal cells of the rabbit gastric gland. J Biol Chem. 1986 Feb 25;261(6):2660–2667. [PubMed] [Google Scholar]
- Muallem S., Schoeffield M., Pandol S., Sachs G. Inositol trisphosphate modification of ion transport in rough endoplasmic reticulum. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4433–4437. doi: 10.1073/pnas.82.13.4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs D. L., Korenbrot J. I., Williams J. A. Intracellular free calcium concentrations in isolated pancreatic acini; effects of secretagogues. Biochem Biophys Res Commun. 1983 Nov 30;117(1):122–128. doi: 10.1016/0006-291x(83)91549-8. [DOI] [PubMed] [Google Scholar]
- Pandol S. J., Schoeffield M. S., Fimmel C. J., Muallem S. The agonist-sensitive calcium pool in the pancreatic acinar cell. Activation of plasma membrane Ca2+ influx mechanism. J Biol Chem. 1987 Dec 15;262(35):16963–16968. [PubMed] [Google Scholar]
- Pandol S. J., Schoeffield M. S., Sachs G., Muallem S. Role of free cytosolic calcium in secretagogue-stimulated amylase release from dispersed acini from guinea pig pancreas. J Biol Chem. 1985 Aug 25;260(18):10081–10086. [PubMed] [Google Scholar]
- Ponnappa B. C., Williams J. A. Comparison of 45Ca2+ uptake activity by microsomes from control and stimulated mouse pancreatic acini. Life Sci. 1981 May 21;28(21):2395–2402. doi: 10.1016/0024-3205(81)90506-3. [DOI] [PubMed] [Google Scholar]
- Richardson A. E., Dormer R. L. Calcium-ion-transporting activity in two microsomal subfractions from rat pancreatic acini. Modulation by carbamylcholine. Biochem J. 1984 Apr 15;219(2):679–685. doi: 10.1042/bj2190679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekar M. C., Dixon J. F., Hokin L. E. The formation of inositol 1,2-cyclic 4,5-trisphosphate and inositol 1,2-cyclic 4-bisphosphate on stimulation of mouse pancreatic minilobules with carbamylcholine. J Biol Chem. 1987 Jan 5;262(1):340–344. [PubMed] [Google Scholar]
- Shears S. B., Storey D. J., Morris A. J., Cubitt A. B., Parry J. B., Michell R. H., Kirk C. J. Dephosphorylation of myo-inositol 1,4,5-trisphosphate and myo-inositol 1,3,4-triphosphate. Biochem J. 1987 Mar 1;242(2):393–402. doi: 10.1042/bj2420393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolze H., Schulz I. Effect of atropine, ouabain, antimycin A, and A23187 on "trigger Ca2+ pool" in exocrine pancreas. Am J Physiol. 1980 Apr;238(4):G338–G348. doi: 10.1152/ajpgi.1980.238.4.G338. [DOI] [PubMed] [Google Scholar]
- Streb H., Bayerdörffer E., Haase W., Irvine R. F., Schulz I. Effect of inositol-1,4,5-trisphosphate on isolated subcellular fractions of rat pancreas. J Membr Biol. 1984;81(3):241–253. doi: 10.1007/BF01868717. [DOI] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Tarver A. P., King W. G., Rittenhouse S. E. Inositol 1,4,5-trisphosphate and inositol 1,2-cyclic 4,5-trisphosphate are minor components of total mass of inositol trisphosphate in thrombin-stimulated platelets. Rapid formation of inositol 1,3,4-trisphosphate. J Biol Chem. 1987 Dec 25;262(36):17268–17271. [PubMed] [Google Scholar]
- Trimble E. R., Bruzzone R., Meehan C. J., Biden T. J. Rapid increases in inositol 1,4,5-trisphosphate, inositol 1,3,4,5-tetrakisphosphate and cytosolic free Ca2+ in agonist-stimulated pancreatic acini of the rat. Effect of carbachol, caerulein and secretin. Biochem J. 1987 Feb 15;242(1):289–292. doi: 10.1042/bj2420289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. B., Connolly T. M., Bross T. E., Majerus P. W., Sherman W. R., Tyler A. N., Rubin L. J., Brown J. E. Isolation and characterization of the inositol cyclic phosphate products of polyphosphoinositide cleavage by phospholipase C. Physiological effects in permeabilized platelets and Limulus photoreceptor cells. J Biol Chem. 1985 Nov 5;260(25):13496–13501. [PubMed] [Google Scholar]


