Abstract
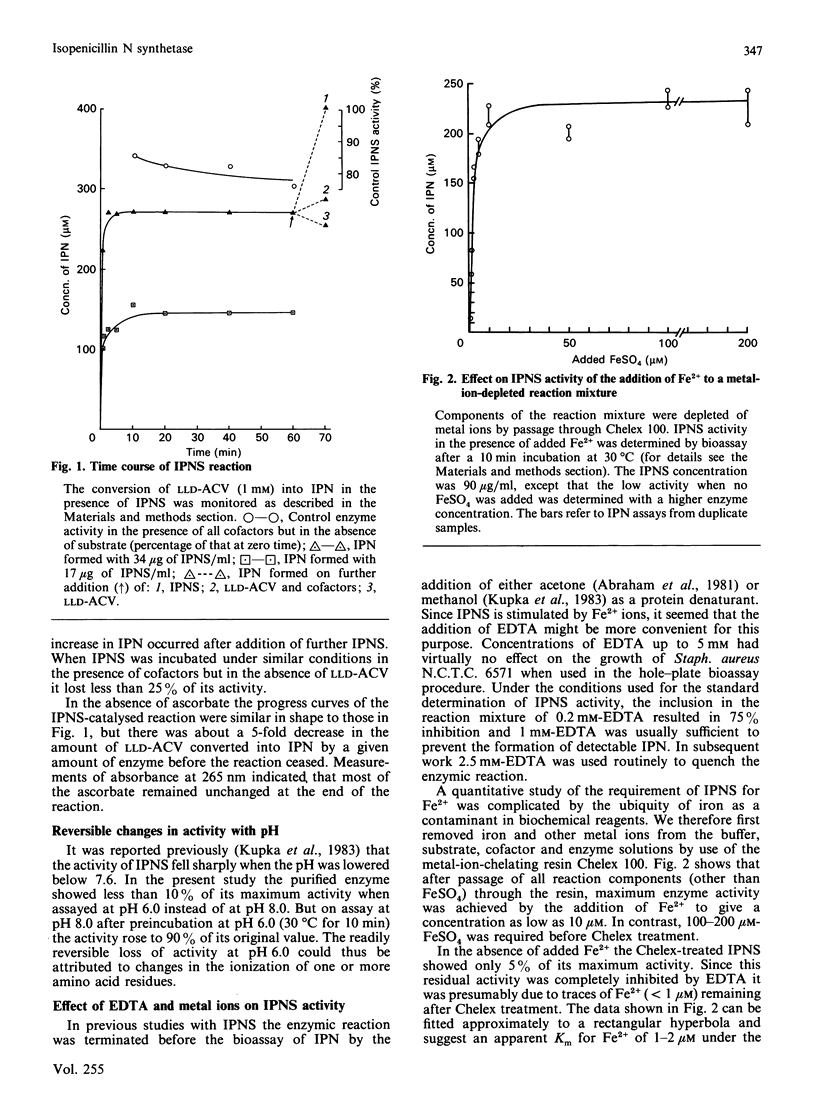
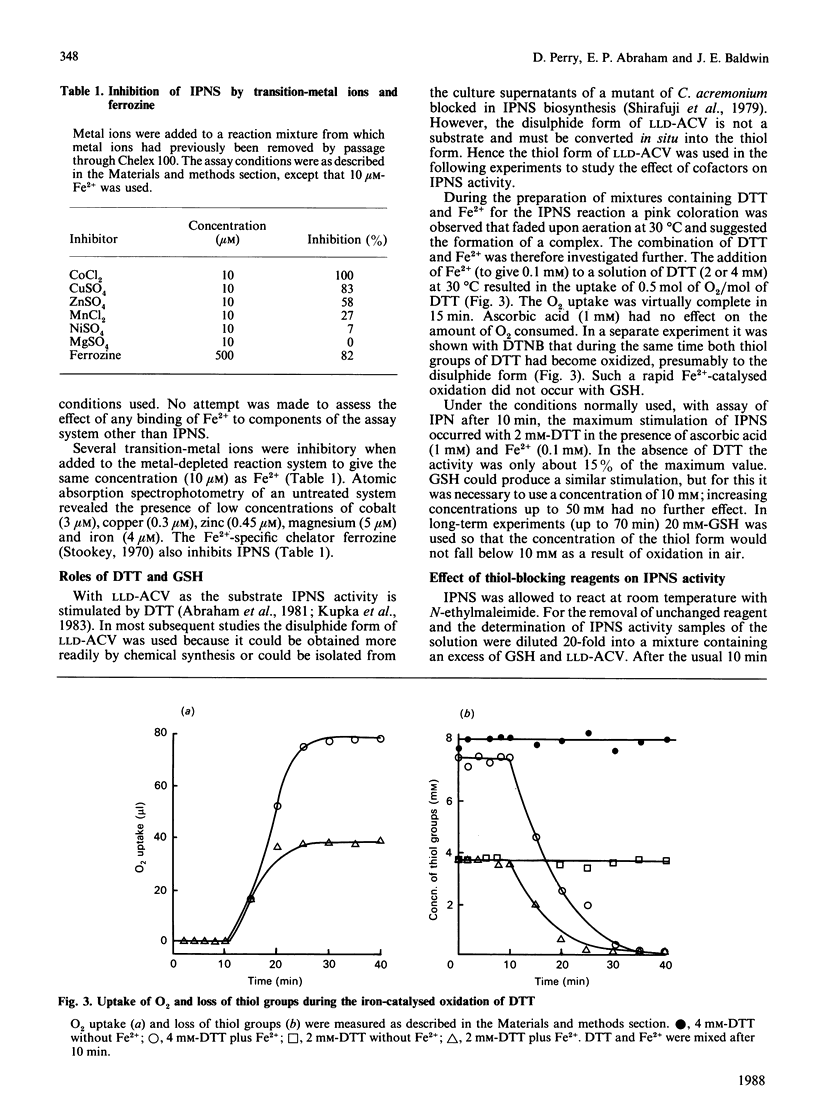
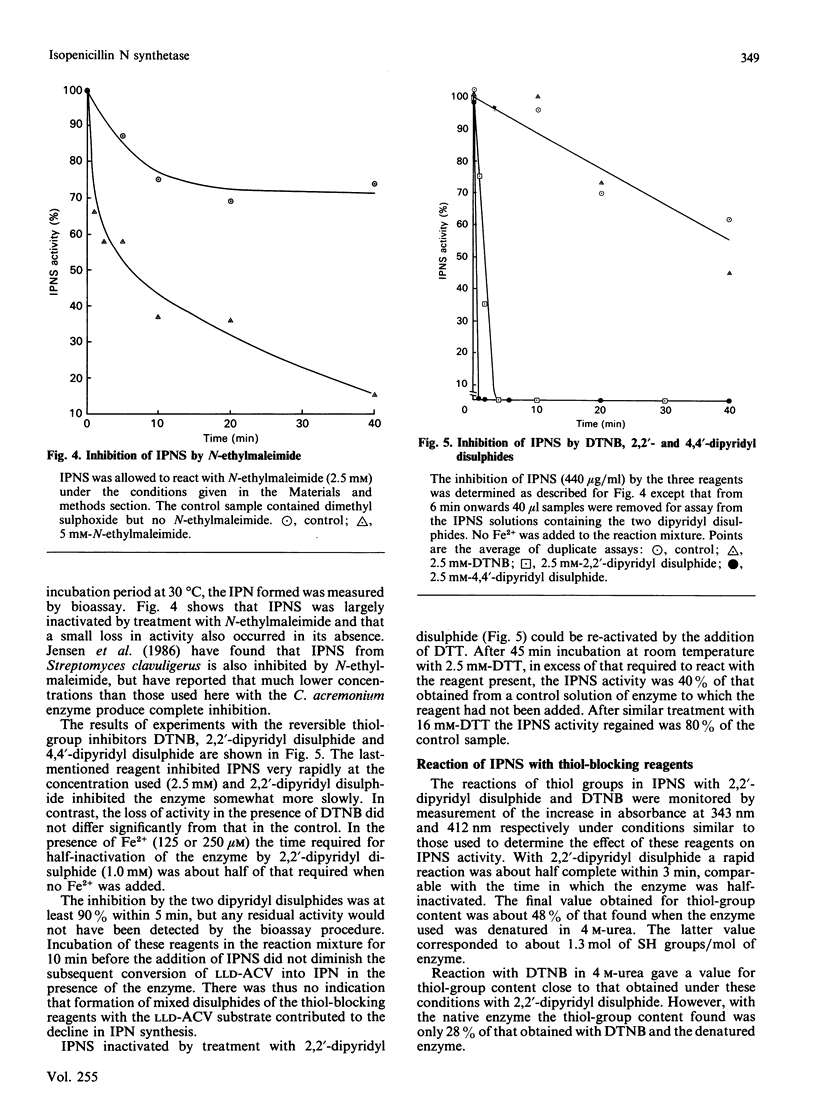
1. Isopenicillin N synthetase (IPNS) from Cephalosporium acremonium, which requires Fe2+ and O2 for activity, was highly purified for studies of factors affecting its conversion of delta-(L-alpha-aminoadipoyl)-L-cysteinyl-D-valine (LLD-ACV) into isopenicillin N (IPN). EDTA was used to quench the reaction by removal of Fe2+. 2. IPNS was inactivated during the course of the conversion of LLD-ACV into IPN, although it was relatively stable in the absence of LLD-ACV under otherwise similar conditions. In the presence of GSH and ascorbate each IPNS molecule carried out about 200 catalytic events before inactivation, but the turnover number was decreased 5-fold in the absence of ascorbate. 3. After trace metal ions had been removed from IPNS and other components of the reaction mixture by Chelex-100 resin, only about 10 microM-Fe2+ was required for maximum stimulation. Several other transition-metal ions were inhibitors of the enzyme. 4. Both dithiothreitol (DTT) and GSH stimulated IPNS activity, but GSH, unlike DTT, was not rapidly oxidized in the presence of O2 and Fe2+. 5. IPNS was rapidly inhibited by the thiol-blocking reagents N-ethylmaleimide and 2,2'- and 4,4'-dipyridyl disulphide, but not by 5,5'-dithiobis-(2-nitrobenzoic acid) in the same concentration. Inhibition by 2,2'-dipyridyl disulphide could be reversed by DTT.
Full text
PDF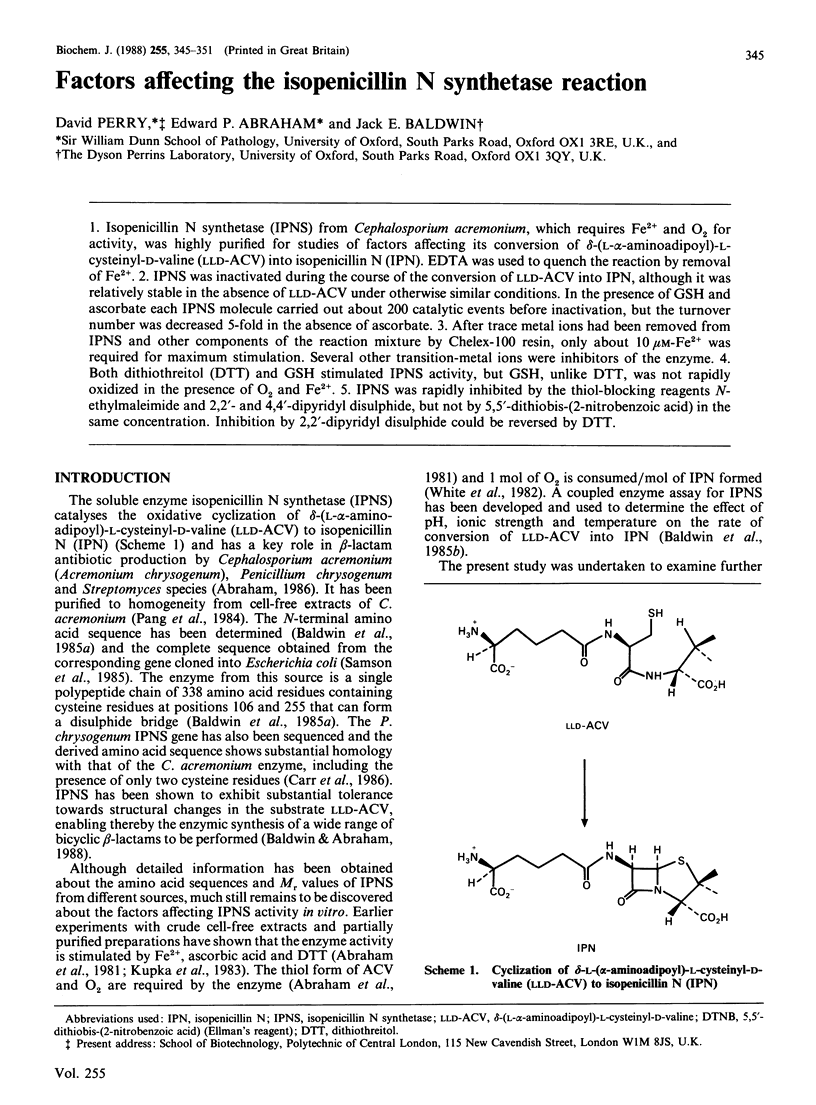



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin J. E., Abraham E. The biosynthesis of penicillins and cephalosporins. Nat Prod Rep. 1988 Apr;5(2):129–145. doi: 10.1039/np9880500129. [DOI] [PubMed] [Google Scholar]
- Baldwin J. E., Gagnon J., Ting H. N-terminal amino acid sequence and some properties of isopenicillin-N synthetase from Cephalosporium acremonium. FEBS Lett. 1985 Sep 2;188(2):253–256. doi: 10.1016/0014-5793(85)80382-3. [DOI] [PubMed] [Google Scholar]
- Baldwin J. E., Moroney S. E., Ting H. H. A coupled enzyme assay for isopenicillin N synthetase. Anal Biochem. 1985 Feb 15;145(1):183–187. doi: 10.1016/0003-2697(85)90345-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Carr L. G., Skatrud P. L., Scheetz M. E., 2nd, Queener S. W., Ingolia T. D. Cloning and expression of the isopenicillin N synthetase gene from Penicillium chrysogenum. Gene. 1986;48(2-3):257–266. doi: 10.1016/0378-1119(86)90084-3. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Jensen S. E., Leskiw B. K., Vining L. C., Aharonowitz Y., Westlake D. W., Wolfe S. Purification of isopenicillin N synthetase from Streptomyces clavuligerus. Can J Microbiol. 1986 Dec;32(12):953–958. doi: 10.1139/m86-176. [DOI] [PubMed] [Google Scholar]
- Kim K., Rhee S. G., Stadtman E. R. Nonenzymatic cleavage of proteins by reactive oxygen species generated by dithiothreitol and iron. J Biol Chem. 1985 Dec 15;260(29):15394–15397. [PubMed] [Google Scholar]
- Kupka J., Shen Y. Q., Wolfe S., Demain A. L. Studies on the ring-cyclization and ring-expansion enzymes of beta-lactam biosynthesis in Cephalosporium acremonium. Can J Microbiol. 1983 May;29(5):488–496. doi: 10.1139/m83-078. [DOI] [PubMed] [Google Scholar]
- Pang C. P., Chakravarti B., Adlington R. M., Ting H. H., White R. L., Jayatilake G. S., Baldwin J. E., Abraham E. P. Purification of isopenicillin N synthetase. Biochem J. 1984 Sep 15;222(3):789–795. doi: 10.1042/bj2220789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson S. M., Belagaje R., Blankenship D. T., Chapman J. L., Perry D., Skatrud P. L., VanFrank R. M., Abraham E. P., Baldwin J. E., Queener S. W. Isolation, sequence determination and expression in Escherichia coli of the isopenicillin N synthetase gene from Cephalosporium acremonium. Nature. 1985 Nov 14;318(6042):191–194. doi: 10.1038/318191a0. [DOI] [PubMed] [Google Scholar]
- Samson S. M., Chapman J. L., Belagaje R., Queener S. W., Ingolia T. D. Analysis of the role of cysteine residues in isopenicillin N synthetase activity by site-directed mutagenesis. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5705–5709. doi: 10.1073/pnas.84.16.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. L., John E. M., Baldwin J. E., Abraham E. P. Stoichiometry of oxygen consumption in the biosynthesis of isopenicillin from a tripeptide. Biochem J. 1982 Jun 1;203(3):791–793. doi: 10.1042/bj2030791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong L., Albracht S. P., Kemp A. Prolyl 4-hydroxylase activity in relation to the oxidation state of enzyme-bound iron. The role of ascorbate in peptidyl proline hydroxylation. Biochim Biophys Acta. 1982 Jun 4;704(2):326–332. doi: 10.1016/0167-4838(82)90162-5. [DOI] [PubMed] [Google Scholar]


