Abstract
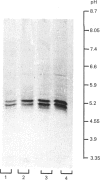
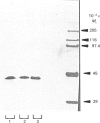
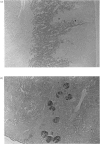
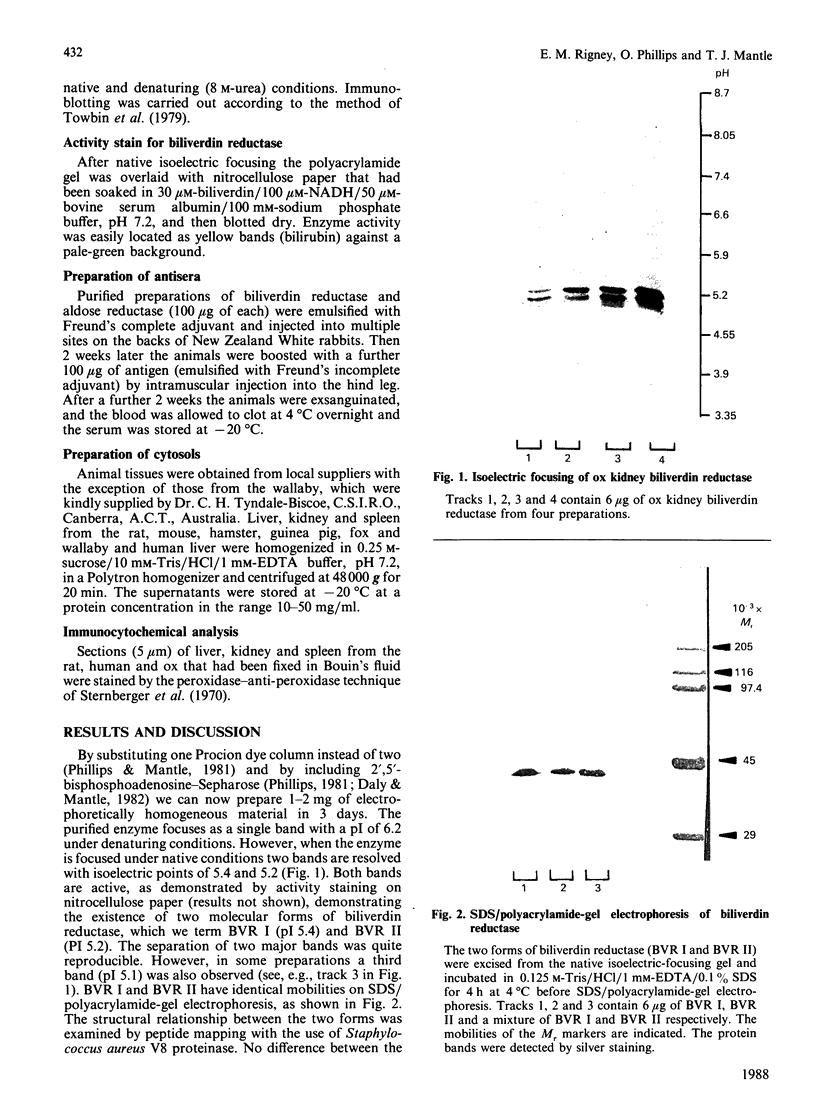
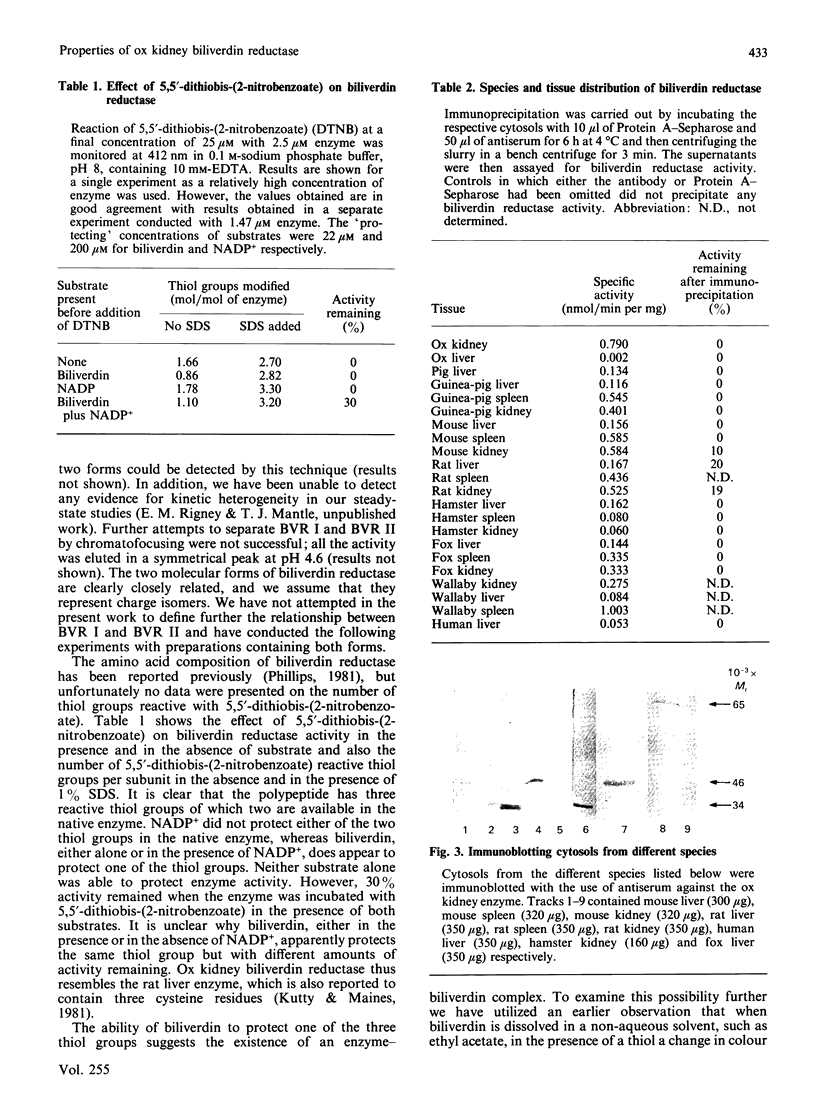
The liver, kidney and spleen of the mouse and rat and the kidney and spleen of the ox express a monomeric form of biliverdin reductase (Mr 34,000), which in the case of the ox kidney enzyme exists in two forms (pI 5.4 and 5.2) that are probably charge isomers. The livers of the mouse and rats express, in addition, a protein (Mr 46,000) that cross-reacts with antibodies raised against the ox kidney enzyme and may be related to form 2 described by Frydman, Tomaro, Awruch & Frydman [(1983) Biochim. Biophys. Acta 759, 257-263]. Higher-Mr forms appear to exist in the guinea pig and hamster. The ox kidney enzyme has three thiol groups, of which two are accessible to 5,5'-dithiobis-(2-nitrobenzoate) in the native enzyme. Immunocytochemical analysis reveals that biliverdin reductase is localized in proximal tubules of the inner cortex of the rat kidney. Biliverdin reductase antiserum also stains proximal tubules in human and ox kidney. The staining of podocytes in glomeruli of ox kidney with antiserum to aldose reductase is particularly prominent. The localization of biliverdin reductase in the inner cortical zone of rat kidney is similar to that described for glutathione S-transferase YfYf, and it is suggested that one function of this 'intracellular binding protein' may be to maintain a low free concentration of biliverdin to allow biliverdin reductase to operate efficiently.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baird J. K., Sherwood R. F., Carr R. J., Atkinson A. Enzyme purification by substrate elution chromatography from procion dye-polysaccharide matrices. FEBS Lett. 1976 Nov;70(1):61–66. doi: 10.1016/0014-5793(76)80726-0. [DOI] [PubMed] [Google Scholar]
- Daly A. K., Mantle T. J. Purification and characterization of the multiple forms of aldehyde reductase in ox kidney. Biochem J. 1982 Aug 1;205(2):373–380. doi: 10.1042/bj2050373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frydman R. B., Tomaro M. L., Awruch J., Frydman B. Interconversion of the molecular forms of biliverdin reductase from rat liver. Biochim Biophys Acta. 1983 Sep 13;759(3):257–263. doi: 10.1016/0304-4165(83)90321-5. [DOI] [PubMed] [Google Scholar]
- Knight B. L., Myant N. B. The effect of noradrenaline on glyceride synthesis and oxidative metabolism in vitro in the brown fat of newborn rabbits. Biochem J. 1971 Nov;125(1):1–8. doi: 10.1042/bj1250001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutty R. K., Maines M. D. Purification and characterization of biliverdin reductase from rat liver. J Biol Chem. 1981 Apr 25;256(8):3956–3962. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ludvigson M. A., Sorenson R. L. Immunohistochemical localization of aldose reductase. II. Rat eye and kidney. Diabetes. 1980 Jun;29(6):450–459. doi: 10.2337/diab.29.6.450. [DOI] [PubMed] [Google Scholar]
- McDonagh A. F., Palma L. A., Schmid R. Reduction of biliverdin and placental transfer of bilirubin and biliverdin in the pregnant guinea pig. Biochem J. 1981 Jan 15;194(1):273–282. doi: 10.1042/bj1940273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi M., Yoshida T., Kikuchi G. Purification and properties of biliverdin reductases from pig spleen and rat liver. J Biochem. 1979 Oct;86(4):833–848. doi: 10.1093/oxfordjournals.jbchem.a132615. [DOI] [PubMed] [Google Scholar]
- Okazaki K., Nishimura H., Arizono H., Nishimura N., Suzuki Y. Biliverdin initiates the liver regeneration in the rat--a hypothesis. Biochem Biophys Res Commun. 1978 Mar 30;81(2):512–520. doi: 10.1016/0006-291x(78)91564-4. [DOI] [PubMed] [Google Scholar]
- Phillips O., Mantle T. J. Some kinetic and physical properties of biliverdin reductase. Biochem Soc Trans. 1981 Aug;9(4):275–278. doi: 10.1042/bst0090275. [DOI] [PubMed] [Google Scholar]
- Phillips O., Mantle T. J., Tuffery A. R., Heyworth C. M., Wilson S. R., Houslay M. D. On the possible role of biliverdin stimulation of cyclic AMP levels as a trigger for liver regeneration in the rat. Biochem Pharmacol. 1984 Jun 15;33(12):1963–1967. doi: 10.1016/0006-2952(84)90556-2. [DOI] [PubMed] [Google Scholar]
- Sternberger L. A., Hardy P. H., Jr, Cuculis J. J., Meyer H. G. The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem. 1970 May;18(5):315–333. doi: 10.1177/18.5.315. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]