Abstract
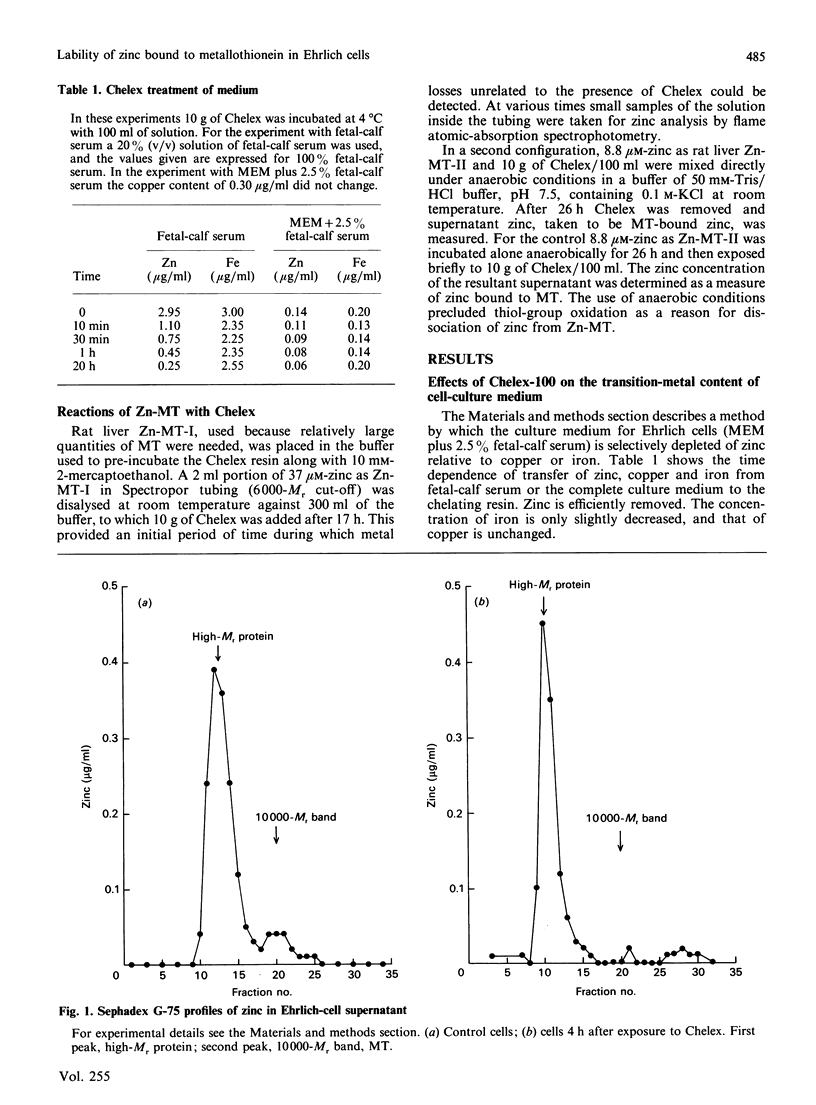
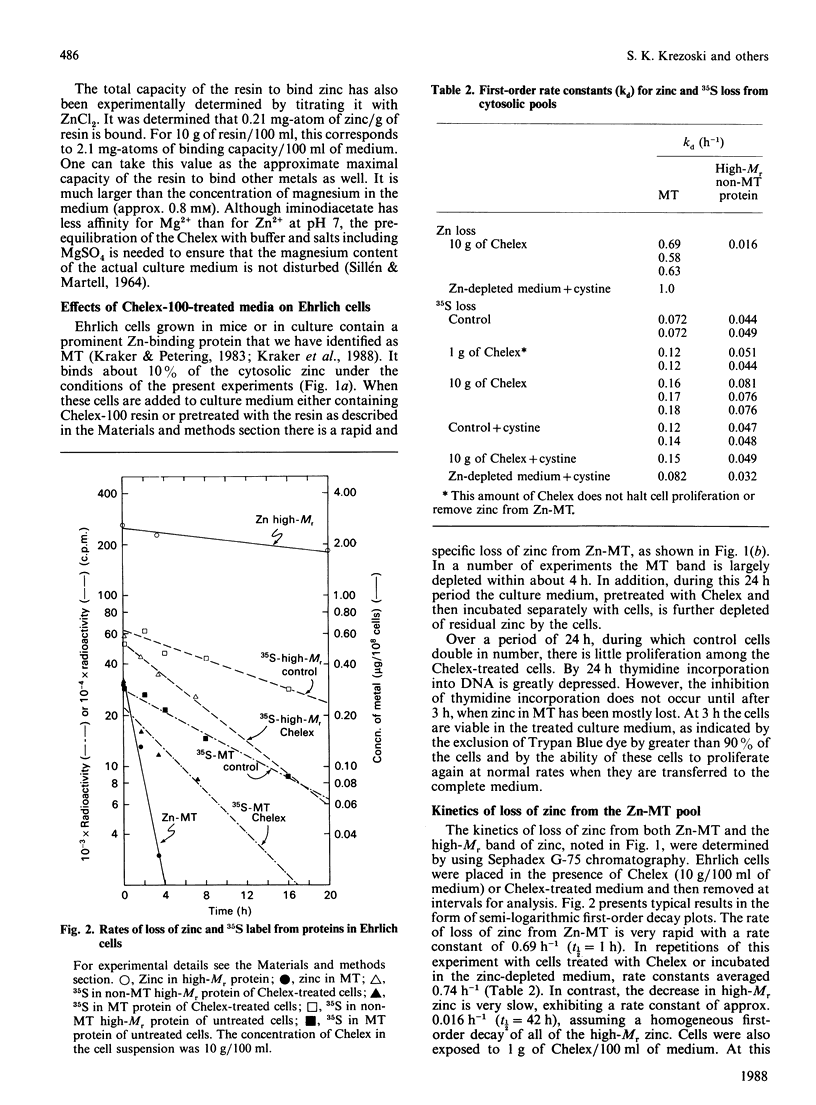
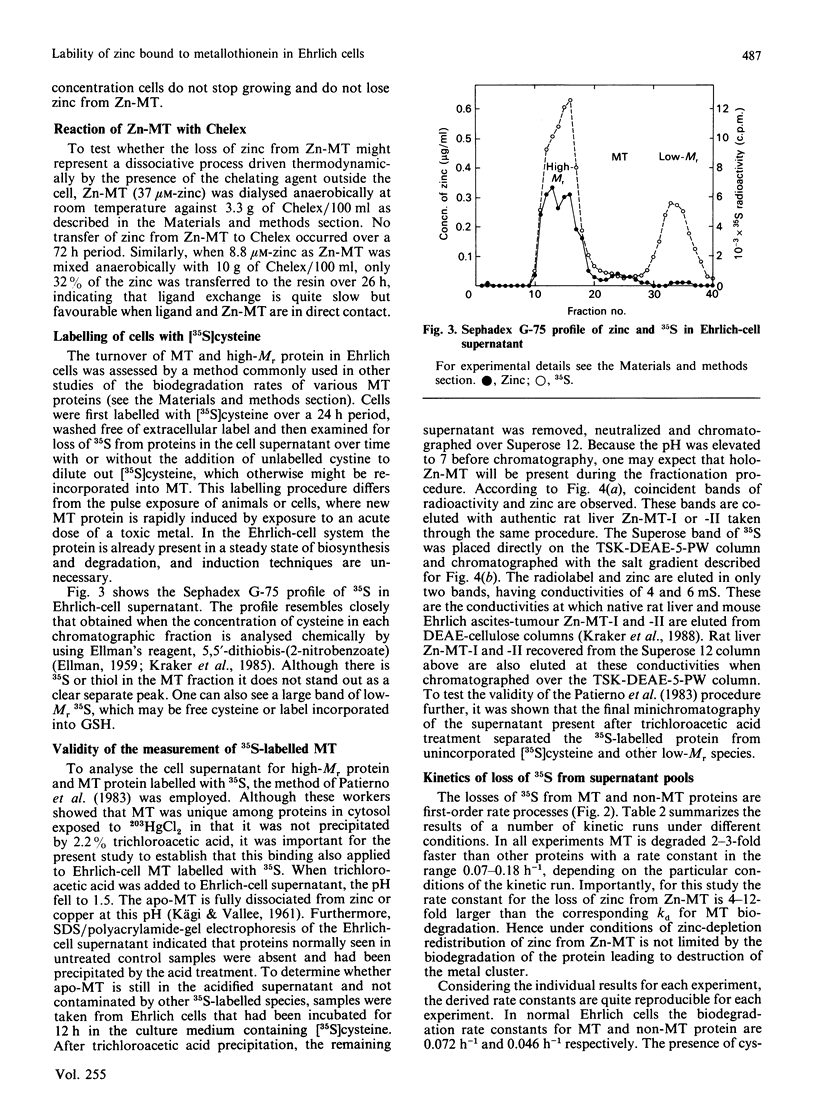
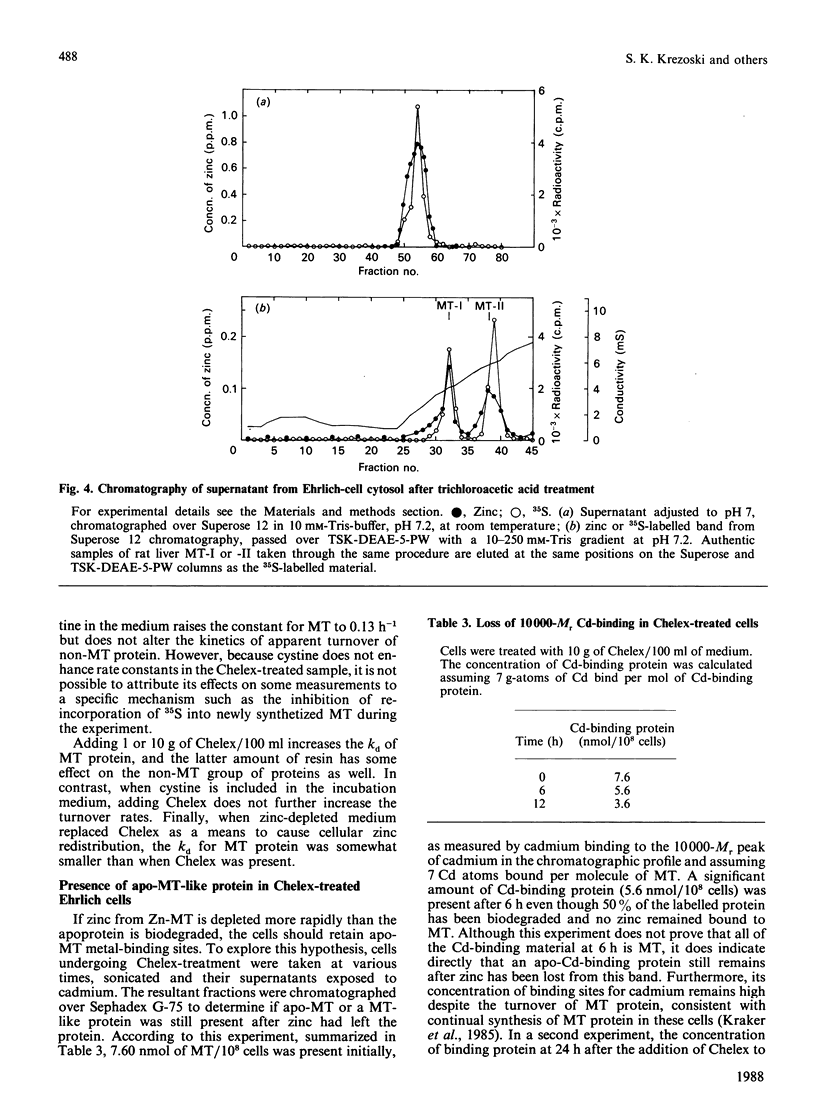
Ehrlich ascites-tumour cells normally contain a large concentration of Zn-metallothionein. When cells are placed in culture media, containing or pretreated with the metal-ion-chelating resin Chelex-100, they stop growing, remain viable and lose zinc specifically from the metallothionein (MT) pool. The kinetics of loss of zinc are first-order and are very rapid, having a rate constant of greater than or equal to 0.6 h-1. MT protein labelled with 35S is biodegraded with a rate constant of 0.07-0.014 h-1 in control cells, 0.08 h-1 in cells exposed to the zinc-deficient medium and 0.12-0.18 h-1 in cells treated directly with Chelex. Over the 6 h period in which zinc is totally lost from Zn-MT there is relatively little decrease in MT-like protein as measured by cadmium-binding to the 10,000-Mr protein fraction. Other pools of zinc and 35S-labelled protein turn over more slowly. There is no loss of zinc from rat liver Zn-MT that is dialysed against Chelex to model the possible reaction of the resin with Ehrlich-cell Zn-MT. However, Chelex does compete slowly for MT-bound zinc when resin and MT are directly mixed. Analysis of the known and possible pathways of zinc metabolism in cells in relationship to these rate constants shows that biodegradation of MT protein cannot account for the rate of loss of zinc from Zn-MT.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bremner I. Involvement of metallothionein in the hepatic metabolism of copper. J Nutr. 1987 Jan;117(1):19–29. doi: 10.1093/jn/117.1.19. [DOI] [PubMed] [Google Scholar]
- Day F. A., Coles B. J., Brady F. O. Postinductive actinomycin D effects on the concentrations of cadmium thionein, zinc thionein, and copper chelatin in rat liver. Bioinorg Chem. 1978;8(2):93–105. doi: 10.1016/s0006-3061(00)80236-7. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Feldman S. L., Cousins R. J. Degradation of hepatic zinc-thionein after parenteral zinc administration. Biochem J. 1976 Dec 15;160(3):583–588. doi: 10.1042/bj1600583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAGI J. H., VALLEE B. L. Metallothionein: a cadmium and zinc-containign protein from equine renal cortex. II. Physico-chemical properties. J Biol Chem. 1961 Sep;236:2435–2442. [PubMed] [Google Scholar]
- Koch J., Wielgus S., Shankara B., Saryan L. A., Shaw C. F., Petering D. H. Zinc-, copper- and cadmium-binding protein in Ehrlich ascites tumour cells. Biochem J. 1980 Jul 1;189(1):95–104. doi: 10.1042/bj1890095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraker A. J., Krakower G., Shaw C. F., 3rd, Petering D. H., Garvey J. S. Zinc metabolism in Ehrlich cells: properties of a metallothionein-like zinc-binding protein. Cancer Res. 1988 Jun 15;48(12):3381–3388. [PubMed] [Google Scholar]
- Kraker A., Krezoski S., Schneider J., Minkel D., Petering D. H. Reaction of 3-ethoxy-2-oxobutyraldehyde bis(thiosemicarbazonato) Cu(II) with Ehrlich cells. Binding of copper to metallothionein and its relationship to zinc metabolism and cell proliferation. J Biol Chem. 1985 Nov 5;260(25):13710–13718. [PubMed] [Google Scholar]
- Krishnamurti C., Saryan L. A., Petering D. H. Effects of ethylenediaminetetraacetic acid and 1,10-phenanthroline on cell proliferation and DNA synthesis of Ehrlich ascites cells. Cancer Res. 1980 Nov;40(11):4092–4099. [PubMed] [Google Scholar]
- Li T. Y., Kraker A. J., Shaw C. F., 3rd, Petering D. H. Ligand substitution reactions of metallothioneins with EDTA and apo-carbonic anhydrase. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6334–6338. doi: 10.1073/pnas.77.11.6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T. Y., Minkel D. T., Shaw C. F., 3rd, Petering D. H. On the reactivity of metallothioneins with 5,5'-dithiobis-(2-nitrobenzoic acid). Biochem J. 1981 Feb 1;193(2):441–446. doi: 10.1042/bj1930441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick C. C., Menard M. P., Cousins R. J. Induction of hepatic metallothionein by feeding zinc to rats of depleted zinc status. Am J Physiol. 1981 Apr;240(4):E414–E421. doi: 10.1152/ajpendo.1981.240.4.E414. [DOI] [PubMed] [Google Scholar]
- Messer H. H., Murray E. J., Goebel N. K. Removal of trace metals from culture media and sera for in vitro deficiency studies. J Nutr. 1982 Apr;112(4):652–657. doi: 10.1093/jn/112.4.652. [DOI] [PubMed] [Google Scholar]
- Minkel D. T., Dolhun P. J., Calhoun B. L., Saryan L. A., Petering D. H. Zinc deficiency and growth of Ehrlich ascites tumor. Cancer Res. 1979 Jul;39(7 Pt 1):2451–2456. [PubMed] [Google Scholar]
- Minkel D. T., Poulsen K., Wielgus S., Shaw C. F., 3rd, Petering D. H. On the sensitivity of metallothioneins to oxidation during isolation. Biochem J. 1980 Nov 1;191(2):475–485. doi: 10.1042/bj1910475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S. H., Deagen J. T., Whanger P. D., Weswig P. H. Biological function of metallothionein-IV. Biosynthesis and degradation of liver and kidney metallothionein in rats fed diets containing zinc or cadmium. Bioinorg Chem. 1978;8(3):245–254. doi: 10.1016/s0006-3061(00)80200-8. [DOI] [PubMed] [Google Scholar]
- Ohtake H., Hasegawa K., Koga M. Zinc-binding protein in the livers of neonatal, normal and partially hepatectomized rats. Biochem J. 1978 Sep 15;174(3):999–1005. doi: 10.1042/bj1740999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onosaka S., Cherian M. G. The induced synthesis of metallothionein in various tissues of rats in response to metals. II. Influence of zinc status and specific effect on pancreatic metallothionein. Toxicology. 1982;23(1):11–20. doi: 10.1016/0300-483x(82)90037-3. [DOI] [PubMed] [Google Scholar]
- Patierno S. R., Pellis N. R., Evans R. M., Costa M. Application of a modified 203Hg binding assay for metallothionein. Life Sci. 1983 Apr 4;32(14):1629–1636. doi: 10.1016/0024-3205(83)90870-6. [DOI] [PubMed] [Google Scholar]
- Petering D. H., Loftsgaarden J., Schneider J., Fowler B. Metabolism of cadmium, zinc and copper in the rat kidney: the role of metallothionein and other binding sites. Environ Health Perspect. 1984 Mar;54:73–81. doi: 10.1289/ehp.845473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saryan L. A., Ankel E., Krishnamurti C., Petering D. H., Elford H. Comparative cytotoxic and biochemical effects of ligands and metal complexes of alpha-N-heterocyclic carboxaldehyde thiosemicarbazones. J Med Chem. 1979 Oct;22(10):1218–1221. doi: 10.1021/jm00196a013. [DOI] [PubMed] [Google Scholar]
- Udom A. O., Brady F. O. Reactivation in vitro of zinc-requiring apo-enzymes by rat liver zinc-thionein. Biochem J. 1980 May 1;187(2):329–335. doi: 10.1042/bj1870329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb M., Cain K. Functions of metallothionein. Biochem Pharmacol. 1982 Jan 15;31(2):137–142. doi: 10.1016/0006-2952(82)90202-7. [DOI] [PubMed] [Google Scholar]


