Abstract
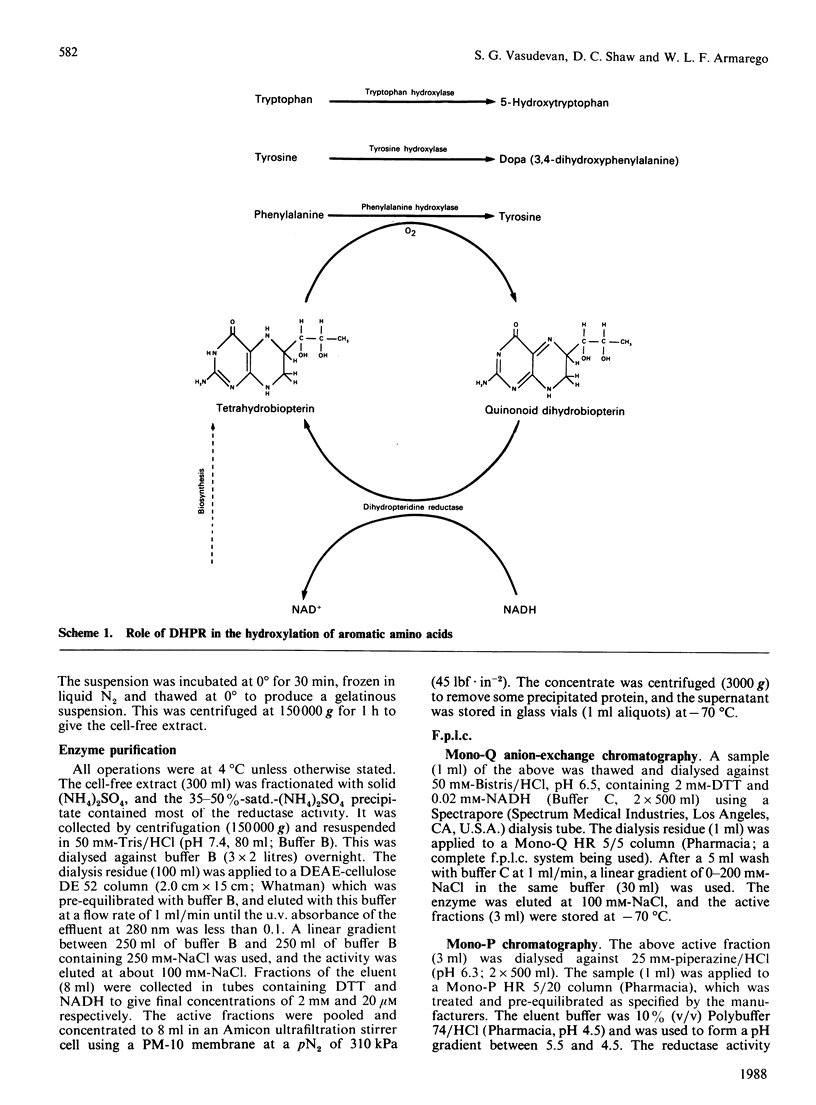
A dihydropteridine reductase from Escherichia coli was purified to apparent homogeneity. It is a dimeric enzyme with identical subunits (Mr 27000) and a free N-terminal group. It can use NADH (Vmax./Km 3.36 s-1) and NADPH (Vmax./Km 1.07 s-1) when 6-methyldihydro-(6H)-pterin is the second substrate, as well as quinonoid dihydro-(6H)-biopterin (Vmax./Km 0.69 s-1), dihydro-(6H)-neopterin (Vmax./Km 0.58 s-1), dihydro-(6H)-monapterin 0.66 s-1), 6-methyldihydro-(6H)-pterin and cis-6,7-dimethyldihydro-(6H)-pterin (Vmax./Km 0.66 s-1) when NADH is the second substrate. The pure reductase has a yellow colour and contains bound FAD. The enzyme also has pterin-independent NADH and NADPH oxidoreductase activities when potassium ferricyanide is the electron acceptor.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aksnes A., Skotland T., Flatmark T., Ljones T. Bovine dihydropteridine reductase: purification by affinity chromatography and comparison of enzymes from liver and adrenal medulla. Neurochem Res. 1979 Jun;4(3):385–398. doi: 10.1007/BF00963808. [DOI] [PubMed] [Google Scholar]
- Armarego W. L., Randles D., Taguchi H. Km and kcat. values for [6,6,7,7-2H]7,8(6H)-dihydropterin and 2,6-diamino-5-iminopyrimidin-4-one with dihydropteridine reductase. Biochem J. 1983 May 1;211(2):357–361. doi: 10.1042/bj2110357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armarego W. L., Randles D., Waring P. Dihydropteridine reductase (DHPR), its cofactors, and its mode of action. Med Res Rev. 1984 Jul-Sep;4(3):267–321. doi: 10.1002/med.2610040302. [DOI] [PubMed] [Google Scholar]
- Armarego W. L., Waring P. Inhibition of human brain dihydropteridine reductase [E.C.1.6.99.10] by the oxidation products of catecholamines, the aminochromes. Biochem Biophys Res Commun. 1983 Jun 29;113(3):895–899. doi: 10.1016/0006-291x(83)91083-5. [DOI] [PubMed] [Google Scholar]
- Baltz R. H., Stonesifer J. Adaptive response and enhancement of N-methyl-N'-nitro-N-nitrosoguanidine mutagenesis by chloramphenicol in Streptomyces fradiae. J Bacteriol. 1985 Nov;164(2):944–946. doi: 10.1128/jb.164.2.944-946.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chauvin M. M., Korri K. K., Tirpak A., Simpson R. C., Scrimgeour K. G. Purification of dihydropterin reductase using immobilized Cibacron Blue. Can J Biochem. 1979 Feb;57(2):178–187. doi: 10.1139/o79-022. [DOI] [PubMed] [Google Scholar]
- Cornish-Bowden A., Endrenyi L. Fitting of enzyme kinetic data without prior knowledge of weights. Biochem J. 1981 Mar 1;193(3):1005–1008. doi: 10.1042/bj1931005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton R. G., Jennings I. A naphthoquinone adsorbent for affinity chromatography of human dihydropteridine reductase. Eur J Biochem. 1978 Feb 1;83(1):319–324. doi: 10.1111/j.1432-1033.1978.tb12097.x. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Firgaira F. A., Choo K. H., Cotton R. G., Danks D. M. Molecular and immunological comparison of human dihydropteridine reductase in liver, cultured fibroblasts and continuous lymphoid cells. Biochem J. 1981 Jul 1;197(1):45–53. doi: 10.1042/bj1970045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firgaira F. A., Cotton R. G., Danks D. M. Isolation and characterization of dihydropteridine reductase from human liver. Biochem J. 1981 Jul 1;197(1):31–43. doi: 10.1042/bj1970031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima T., Nixon J. C. Analysis of reduced forms of biopterin in biological tissues and fluids. Anal Biochem. 1980 Feb;102(1):176–188. doi: 10.1016/0003-2697(80)90336-x. [DOI] [PubMed] [Google Scholar]
- Guroff G., Rhoads C. A. Phenylalanine hydroxylation by Pseudomonas species (ATCC 11299a). Nature of the cofactor. J Biol Chem. 1969 Jan 10;244(1):142–146. [PubMed] [Google Scholar]
- KAUFMAN S. On the structure of the phenylalanine hydroxylation cofactor. J Biol Chem. 1962 Aug;237:2712–2713. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- Rembold H., Buff K. Tetrahydrobiopterin, a cofactor in mitochondrial electron transfer. Effect of tetrahydropterins on intact rat-liver mitochondria. Eur J Biochem. 1972 Aug 4;28(4):579–585. doi: 10.1111/j.1432-1033.1972.tb01946.x. [DOI] [PubMed] [Google Scholar]
- Singer S., Ferone R., Walton L., Elwell L. Isolation of a dihydrofolate reductase-deficient mutant of Escherichia coli. J Bacteriol. 1985 Oct;164(1):470–472. doi: 10.1128/jb.164.1.470-472.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder F., Malone B., Piantadosi C. Tetrahydropteridine-dependent cleavage enzyme for O-alkyl lipids: substrate specificity. Biochim Biophys Acta. 1973 Aug 23;316(2):259–265. doi: 10.1016/0005-2760(73)90018-0. [DOI] [PubMed] [Google Scholar]
- Webber S., Deits T. L., Snyder W. R., Whiteley J. M. The purification of rat and sheep liver dihydropteridine reductases by affinity chromatography on methotrexate-sepharose. Anal Biochem. 1978 Feb;84(2):491–503. doi: 10.1016/0003-2697(78)90068-4. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Williams C. D., Dickens G., Letendre C. H., Guroff G., Haines C., Shiota T. Isolation and characterization of dihydropteridine reductase from Pseudomonas species. J Bacteriol. 1976 Sep;127(3):1197–1207. doi: 10.1128/jb.127.3.1197-1207.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]



