Abstract
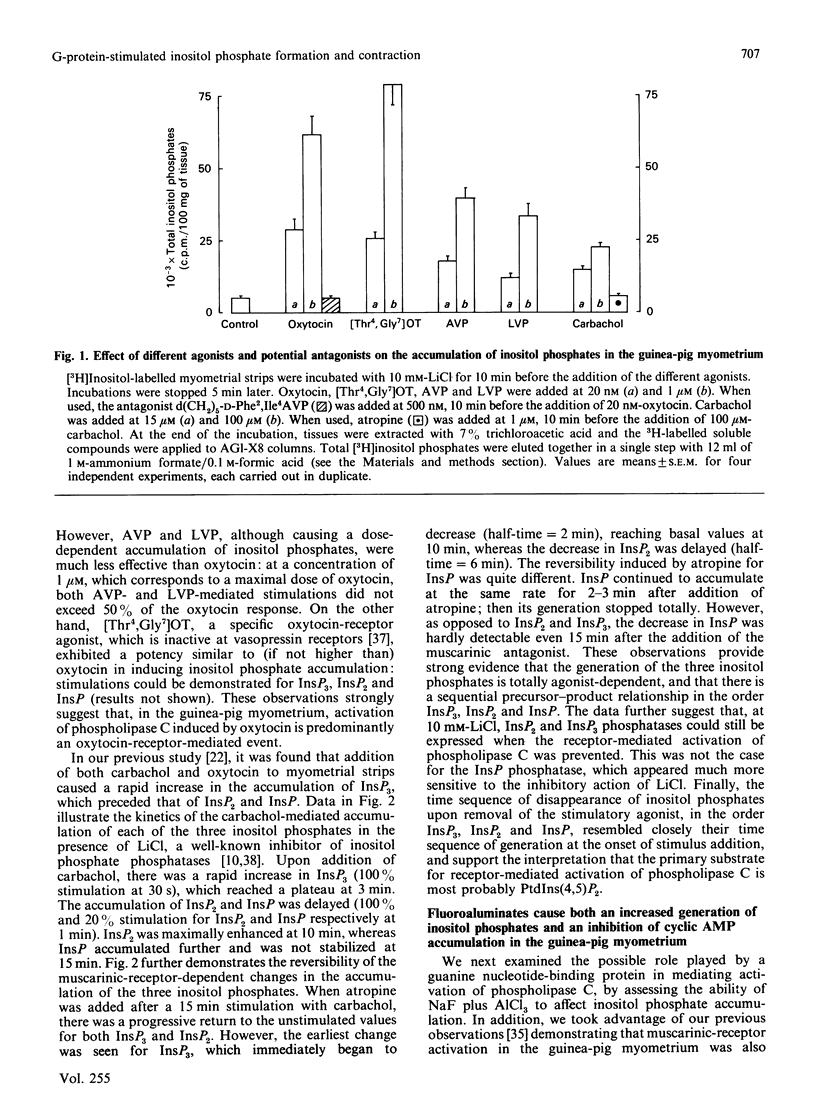
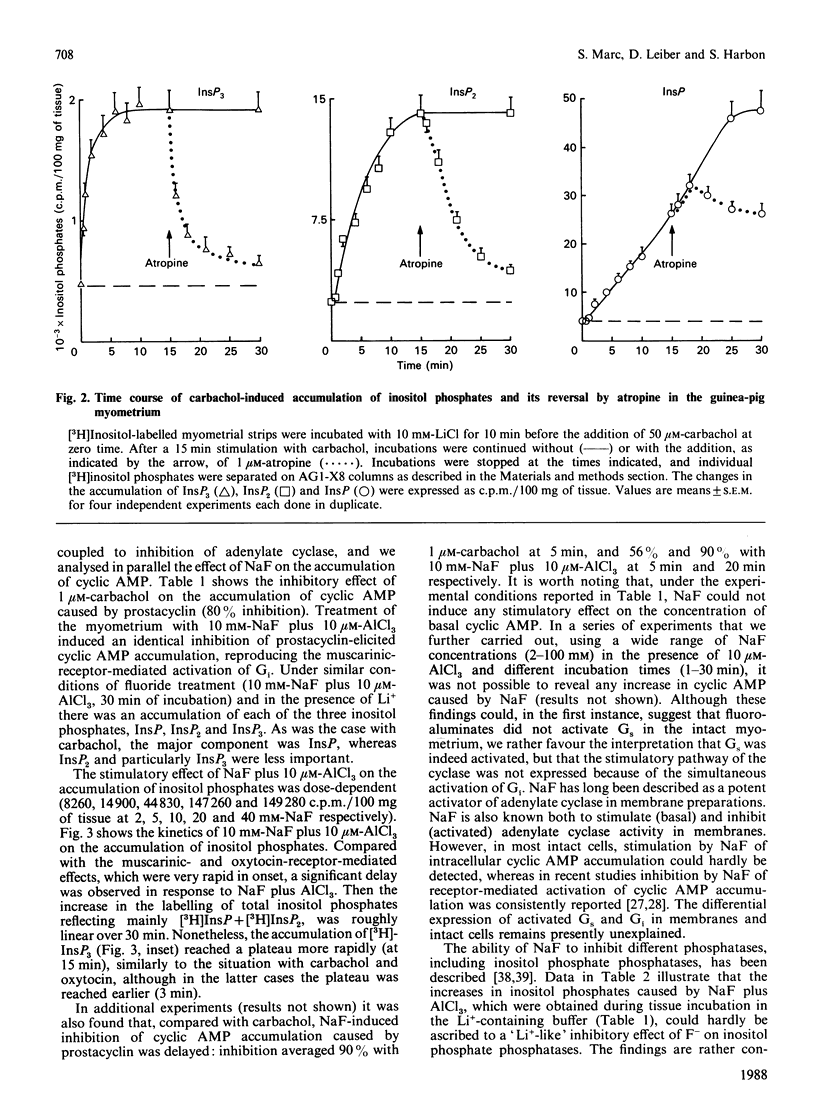
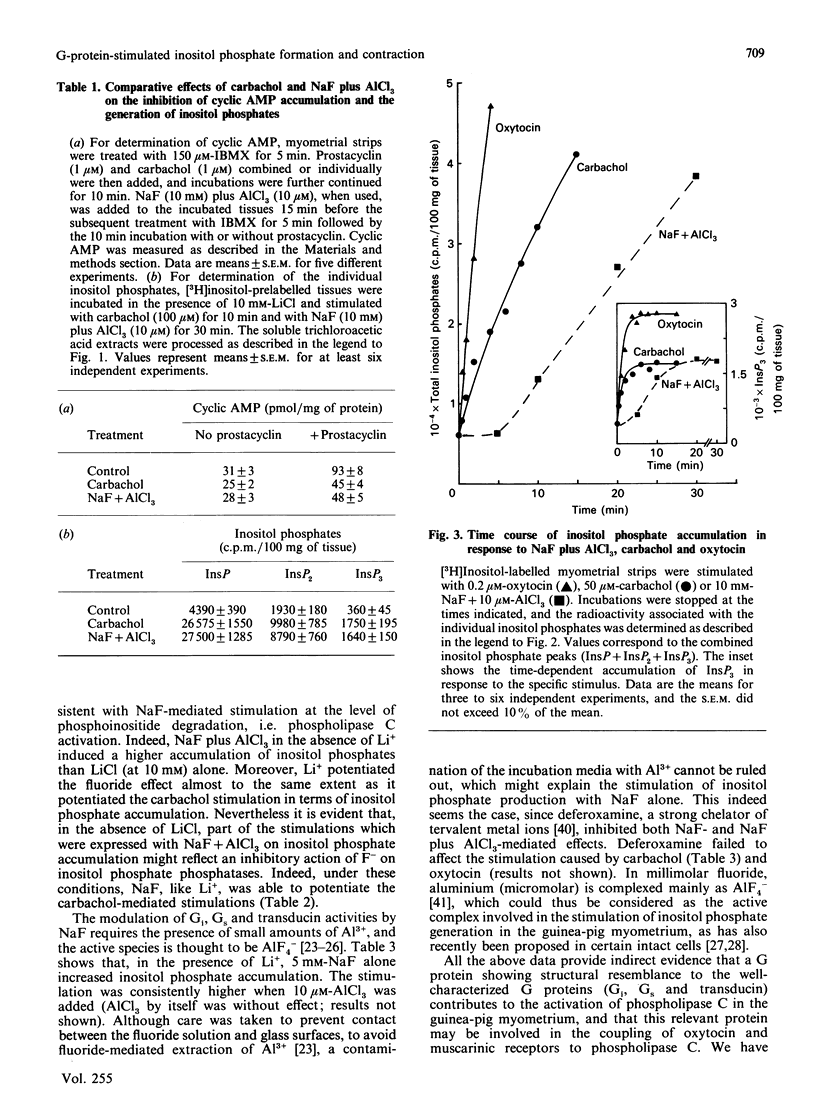
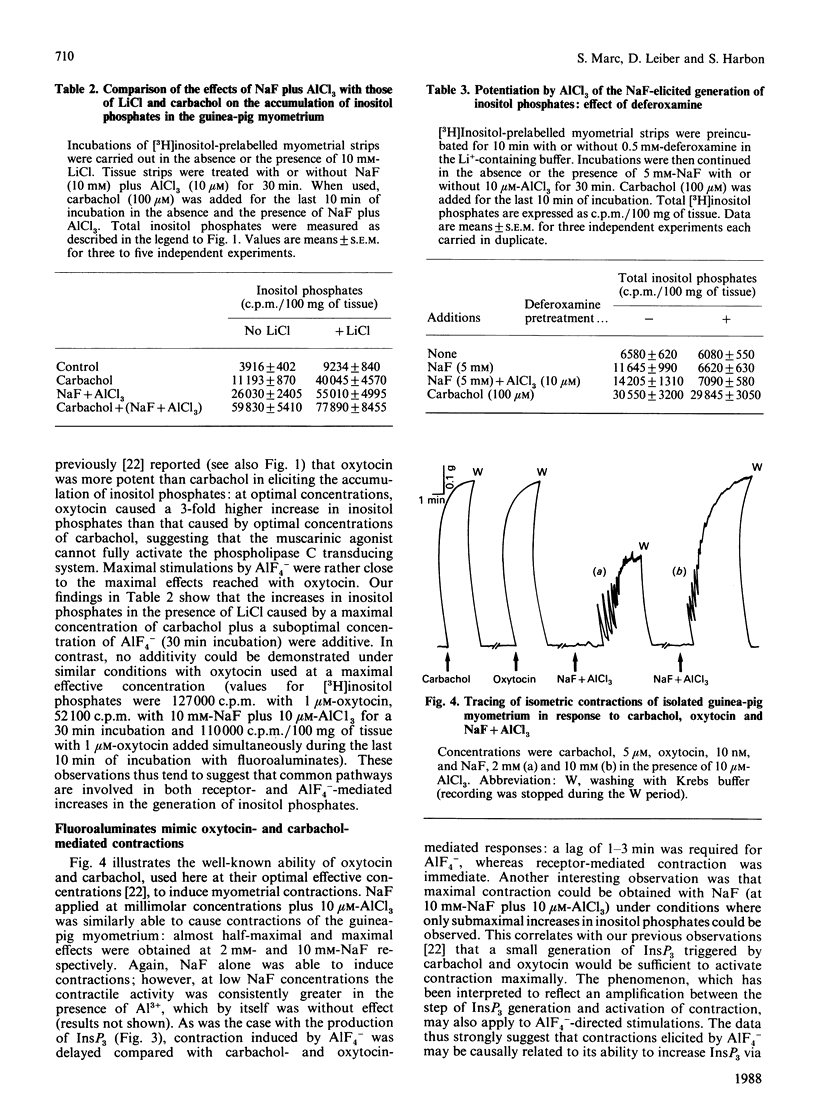
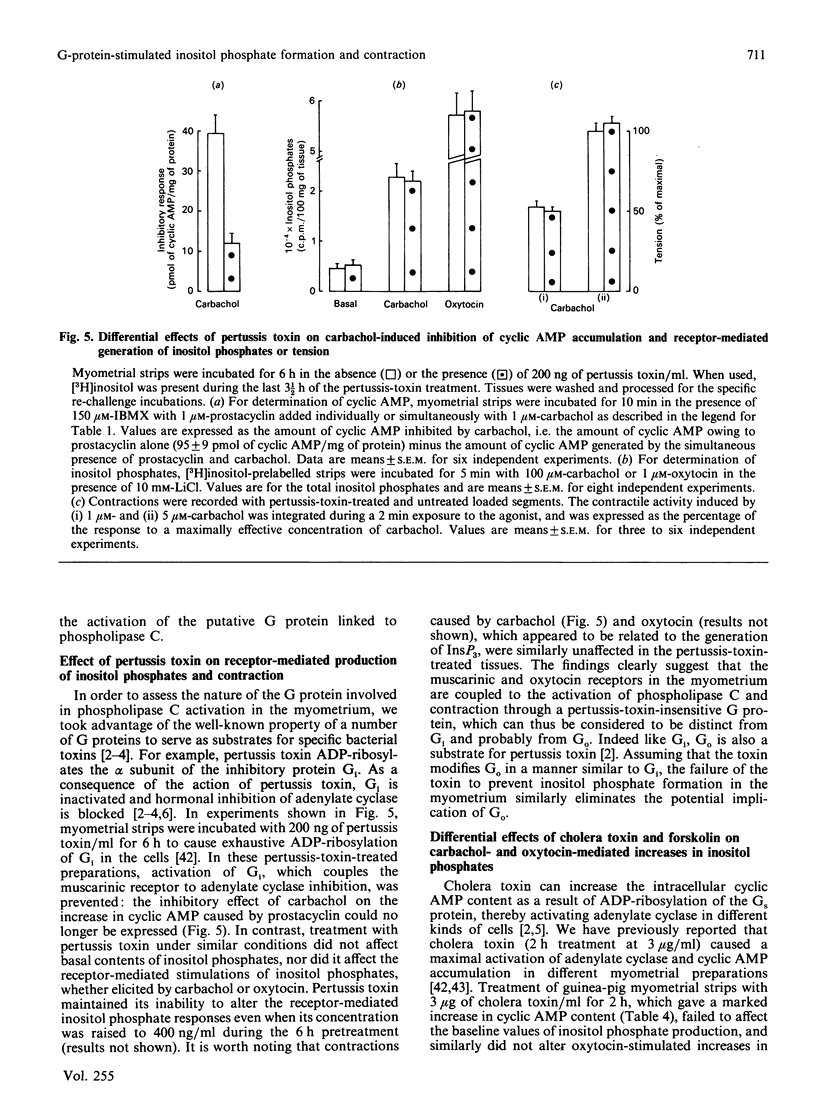
1. In the intact guinea-pig myometrium, carbachol and oxytocin stimulated a specific receptor-mediated phospholipase C activation, catalysing the breakdown of PtdIns(4,5)P2 with the sequential generation of InsP3, InsP2 and InsP. Stimulation of muscarinic receptors also triggered an inhibition of cyclic AMP accumulation caused by prostacyclin. 2. NaF plus AlCl3 mimicked the effects of carbachol and oxytocin by inducing, in a dose-dependent manner, the generation of all three inositol phosphates as well as uterine contractions. AlCl3 enhanced the fluoride effect, supporting the concept that A1F4- was the active species. Under similar conditions, fluoroaluminates activated the guanine nucleotide regulatory protein Gi, reproducing the inhibitory effect of carbachol on cyclic AMP concentrations. 3. Both carbachol- and oxytocin-mediated increases in inositol phosphates, as well as contractions, were insensitive to pertussis toxin, under conditions where the expression of Gi was totally prevented. Cholera toxin, which activates Gs and enhances cyclic AMP accumulation, failed to affect basal or oxytocin-evoked inositol phosphate generation, but induced a slight, though consistent, attenuation of the muscarinic inositol phosphate response, which was similarly evoked by forskolin. 4. The data provide evidence that, in the myometrium, (a) a G protein mediates the generation of inositol phosphates and the Ca2+-dependent contractile event, (b) the relevant G protein that most probably couples muscarinic and oxytocin receptors to phospholipase C is different from Gi and Gs, the proteins that couple receptors to adenylate cyclase, and (c) cyclic AMP does not seem to control the phosphoinositide cycle, but rather exerts a negative regulation at the muscarinic-receptor level.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackrill P., Day J. P. Therapy of aluminium overload (II). Contrib Nephrol. 1984;38:78–80. doi: 10.1159/000408069. [DOI] [PubMed] [Google Scholar]
- Aub D. L., Frey E. A., Sekura R. D., Cote T. E. Coupling of the thyrotropin-releasing hormone receptor to phospholipase C by a GTP-binding protein distinct from the inhibitory or stimulatory GTP-binding protein. J Biol Chem. 1986 Jul 15;261(20):9333–9340. [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bigay J., Deterre P., Pfister C., Chabre M. Fluoride complexes of aluminium or beryllium act on G-proteins as reversibly bound analogues of the gamma phosphate of GTP. EMBO J. 1987 Oct;6(10):2907–2913. doi: 10.1002/j.1460-2075.1987.tb02594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmore P. F., Bocckino S. B., Waynick L. E., Exton J. H. Role of a guanine nucleotide-binding regulatory protein in the hydrolysis of hepatocyte phosphatidylinositol 4,5-bisphosphate by calcium-mobilizing hormones and the control of cell calcium. Studies utilizing aluminum fluoride. J Biol Chem. 1985 Nov 25;260(27):14477–14483. [PubMed] [Google Scholar]
- Bradford P. G., Rubin R. P. Pertussis toxin inhibits chemotactic factor-induced phospholipase C stimulation and lysosomal enzyme secretion in rabbit neutrophils. FEBS Lett. 1985 Apr 22;183(2):317–320. doi: 10.1016/0014-5793(85)80801-2. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. Role of guanine nucleotide binding protein in the activation of polyphosphoinositide phosphodiesterase. Nature. 1985 Apr 11;314(6011):534–536. doi: 10.1038/314534a0. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Graff I., Mockel J., Laurent E., Erneux C., Dumont J. E. Carbachol and sodium fluoride, but not TSH, stimulate the generation of inositol phosphates in the dog thyroid. FEBS Lett. 1987 Jan 5;210(2):204–210. doi: 10.1016/0014-5793(87)81338-8. [DOI] [PubMed] [Google Scholar]
- Hallcher L. M., Sherman W. R. The effects of lithium ion and other agents on the activity of myo-inositol-1-phosphatase from bovine brain. J Biol Chem. 1980 Nov 25;255(22):10896–10901. [PubMed] [Google Scholar]
- Haslam R. J., Davidson M. M. Receptor-induced diacylglycerol formation in permeabilized platelets; possible role for a GTP-binding protein. J Recept Res. 1984;4(1-6):605–629. doi: 10.3109/10799898409042576. [DOI] [PubMed] [Google Scholar]
- Imboden J. B., Shoback D. M., Pattison G., Stobo J. D. Cholera toxin inhibits the T-cell antigen receptor-mediated increases in inositol trisphosphate and cytoplasmic free calcium. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5673–5677. doi: 10.1073/pnas.83.15.5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaho Y., Moss J., Vaughan M. Mechanism of inhibition of transducin GTPase activity by fluoride and aluminum. J Biol Chem. 1985 Sep 25;260(21):11493–11497. [PubMed] [Google Scholar]
- Katada T., Northup J. K., Bokoch G. M., Ui M., Gilman A. G. The inhibitory guanine nucleotide-binding regulatory component of adenylate cyclase. Subunit dissociation and guanine nucleotide-dependent hormonal inhibition. J Biol Chem. 1984 Mar 25;259(6):3578–3585. [PubMed] [Google Scholar]
- Katada T., Ui M. ADP ribosylation of the specific membrane protein of C6 cells by islet-activating protein associated with modification of adenylate cyclase activity. J Biol Chem. 1982 Jun 25;257(12):7210–7216. [PubMed] [Google Scholar]
- Kwatra M. M., Hosey M. M. Phosphorylation of the cardiac muscarinic receptor in intact chick heart and its regulation by a muscarinic agonist. J Biol Chem. 1986 Sep 25;261(27):12429–12432. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leiber D., Harbon S., Guillet J. G., André C., Strosberg A. D. Monoclonal antibodies to purified muscarinic receptor display agonist-like activity. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4331–4334. doi: 10.1073/pnas.81.14.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiber D., Harbon S. The relationship between the carbachol stimulatory effect on cyclic GMP content and activation by fatty acid hydroperoxides of a soluble guanylate cyclase in the guinea pig myometrium. Mol Pharmacol. 1982 May;21(3):654–663. [PubMed] [Google Scholar]
- Litosch I., Wallis C., Fain J. N. 5-Hydroxytryptamine stimulates inositol phosphate production in a cell-free system from blowfly salivary glands. Evidence for a role of GTP in coupling receptor activation to phosphoinositide breakdown. J Biol Chem. 1985 May 10;260(9):5464–5471. [PubMed] [Google Scholar]
- Lowbridge J., Manning M., Haldar J., Sawyer W. H. Synthesis and some pharmacological properties of [4-threonine, 7-glycine]oxytocin, [1-(L-2-hydroxy-3-mercaptopropanoic acid), 4-threonine, 7-glycine]oxytocin (hydroxy[Thr4, Gly7]oxytocin), and [7-Glycine]oxytocin, peptides with high oxytocic-antidiuretic selectivity. J Med Chem. 1977 Jan;20(1):120–123. doi: 10.1021/jm00211a025. [DOI] [PubMed] [Google Scholar]
- Manning M., Olma A., Klis W. A., Seto J., Sawyer W. H. Potent antagonists of the antidiuretic responses to arginine-vasopressin based on modifications of [1-(beta-mercapto-beta,beta-cyclopentamethylenepropionic acid),2-D- phenylalanine,4-valine]arginine-vasopressin at position 4. J Med Chem. 1983 Nov;26(11):1607–1613. doi: 10.1021/jm00365a011. [DOI] [PubMed] [Google Scholar]
- Marc S., Leiber D., Harbon S. Carbachol and oxytocin stimulate the generation of inositol phosphates in the guinea pig myometrium. FEBS Lett. 1986 May 26;201(1):9–14. doi: 10.1016/0014-5793(86)80561-0. [DOI] [PubMed] [Google Scholar]
- Masters S. B., Martin M. W., Harden T. K., Brown J. H. Pertussis toxin does not inhibit muscarinic-receptor-mediated phosphoinositide hydrolysis or calcium mobilization. Biochem J. 1985 May 1;227(3):933–937. doi: 10.1042/bj2270933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt J. E., Taylor C. W., Rubin R. P., Putney J. W., Jr Evidence suggesting that a novel guanine nucleotide regulatory protein couples receptors to phospholipase C in exocrine pancreas. Biochem J. 1986 Jun 1;236(2):337–343. doi: 10.1042/bj2360337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokhtari A., Do Khac L., Tanfin Z., Harbon S. Forskolin modulates cyclic AMP generation in the rat myometrium. Interactions with isoproterenol and prostaglandins E2 and I2. J Cyclic Nucleotide Protein Phosphor Res. 1985;10(3):213–227. [PubMed] [Google Scholar]
- Nakamura T., Ui M. Simultaneous inhibitions of inositol phospholipid breakdown, arachidonic acid release, and histamine secretion in mast cells by islet-activating protein, pertussis toxin. A possible involvement of the toxin-specific substrate in the Ca2+-mobilizing receptor-mediated biosignaling system. J Biol Chem. 1985 Mar 25;260(6):3584–3593. [PubMed] [Google Scholar]
- Neer E. J., Lok J. M., Wolf L. G. Purification and properties of the inhibitory guanine nucleotide regulatory unit of brain adenylate cyclase. J Biol Chem. 1984 Nov 25;259(22):14222–14229. [PubMed] [Google Scholar]
- Paris S., Pouysségur J. Further evidence for a phospholipase C-coupled G protein in hamster fibroblasts. Induction of inositol phosphate formation by fluoroaluminate and vanadate and inhibition by pertussis toxin. J Biol Chem. 1987 Feb 15;262(5):1970–1976. [PubMed] [Google Scholar]
- Sasaguri T., Hirata M., Itoh T., Koga T., Kuriyama H. Guanine nucleotide binding protein involved in muscarinic responses in the pig coronary artery is insensitive to islet-activating protein. Biochem J. 1986 Nov 1;239(3):567–574. doi: 10.1042/bj2390567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W. Forskolin: its biological and chemical properties. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1986;20:1–150. [PubMed] [Google Scholar]
- Spiegel A. M. Signal transduction by guanine nucleotide binding proteins. Mol Cell Endocrinol. 1987 Jan;49(1):1–16. doi: 10.1016/0303-7207(87)90058-x. [DOI] [PubMed] [Google Scholar]
- Sternweis P. C., Gilman A. G. Aluminum: a requirement for activation of the regulatory component of adenylate cyclase by fluoride. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4888–4891. doi: 10.1073/pnas.79.16.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternweis P. C., Robishaw J. D. Isolation of two proteins with high affinity for guanine nucleotides from membranes of bovine brain. J Biol Chem. 1984 Nov 25;259(22):13806–13813. [PubMed] [Google Scholar]
- Storey D. J., Shears S. B., Kirk C. J., Michell R. H. Stepwise enzymatic dephosphorylation of inositol 1,4,5-trisphosphate to inositol in liver. Nature. 1984 Nov 22;312(5992):374–376. doi: 10.1038/312374a0. [DOI] [PubMed] [Google Scholar]
- Stryer L., Bourne H. R. G proteins: a family of signal transducers. Annu Rev Cell Biol. 1986;2:391–419. doi: 10.1146/annurev.cb.02.110186.002135. [DOI] [PubMed] [Google Scholar]
- Stryer L. Cyclic GMP cascade of vision. Annu Rev Neurosci. 1986;9:87–119. doi: 10.1146/annurev.ne.09.030186.000511. [DOI] [PubMed] [Google Scholar]
- Takenawa T., Ishitoya J., Nagai Y. Inhibitory effect of prostaglandin E2, forskolin, and dibutyryl cAMP on arachidonic acid release and inositol phospholipid metabolism in guinea pig neutrophils. J Biol Chem. 1986 Jan 25;261(3):1092–1098. [PubMed] [Google Scholar]
- Tanfin Z., Harbon S. Heterologous regulations of cAMP responses in pregnant rat myometrium. Evolution from a stimulatory to an inhibitory prostaglandin E2 and prostacyclin effect. Mol Pharmacol. 1987 Aug;32(1):249–257. [PubMed] [Google Scholar]
- Thomas A. P., Alexander J., Williamson J. R. Relationship between inositol polyphosphate production and the increase of cytosolic free Ca2+ induced by vasopressin in isolated hepatocytes. J Biol Chem. 1984 May 10;259(9):5574–5584. [PubMed] [Google Scholar]
- Vesin M. F., Harbon S. The effects of epinephrine, prostaglandins, and their antagonists on adenosine cyclic 3',5'-monophosphate concentrations and motility of the rat uterus. Mol Pharmacol. 1974 May;10(3):457–473. [PubMed] [Google Scholar]
- Watson S. P., McConnell R. T., Lapetina E. G. The rapid formation of inositol phosphates in human platelets by thrombin is inhibited by prostacyclin. J Biol Chem. 1984 Nov 10;259(21):13199–13203. [PubMed] [Google Scholar]


