Abstract
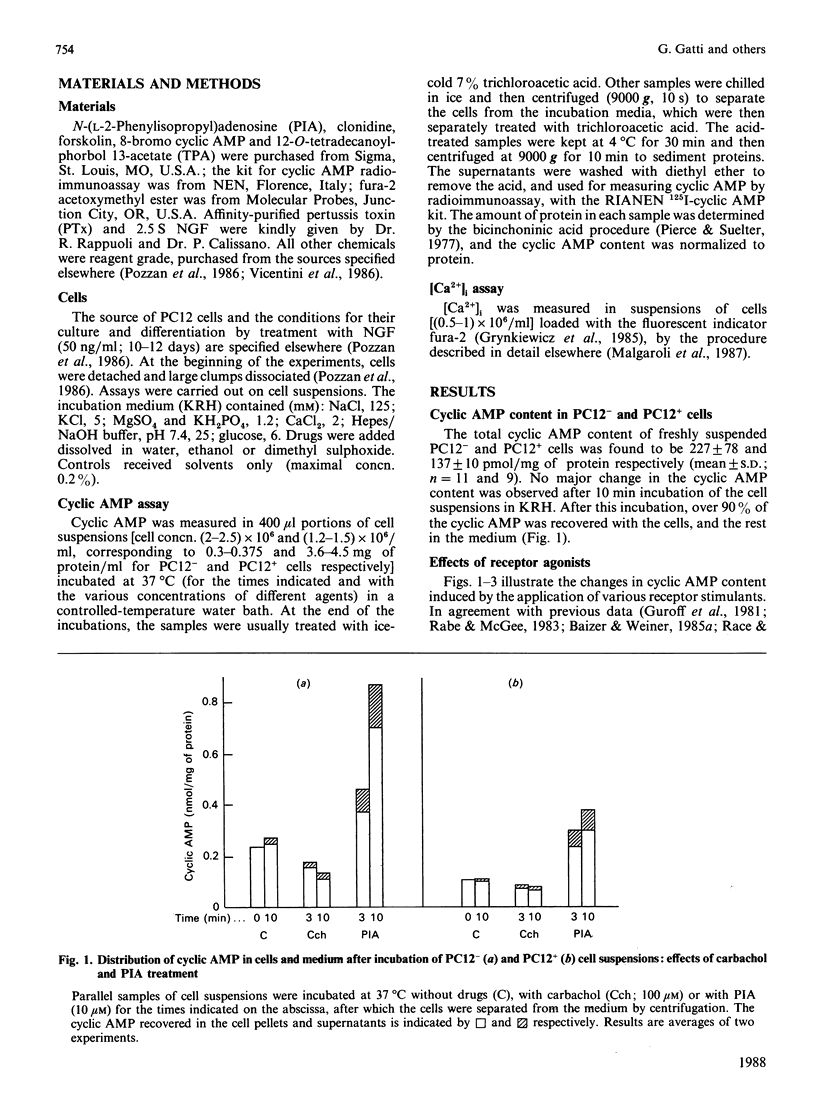
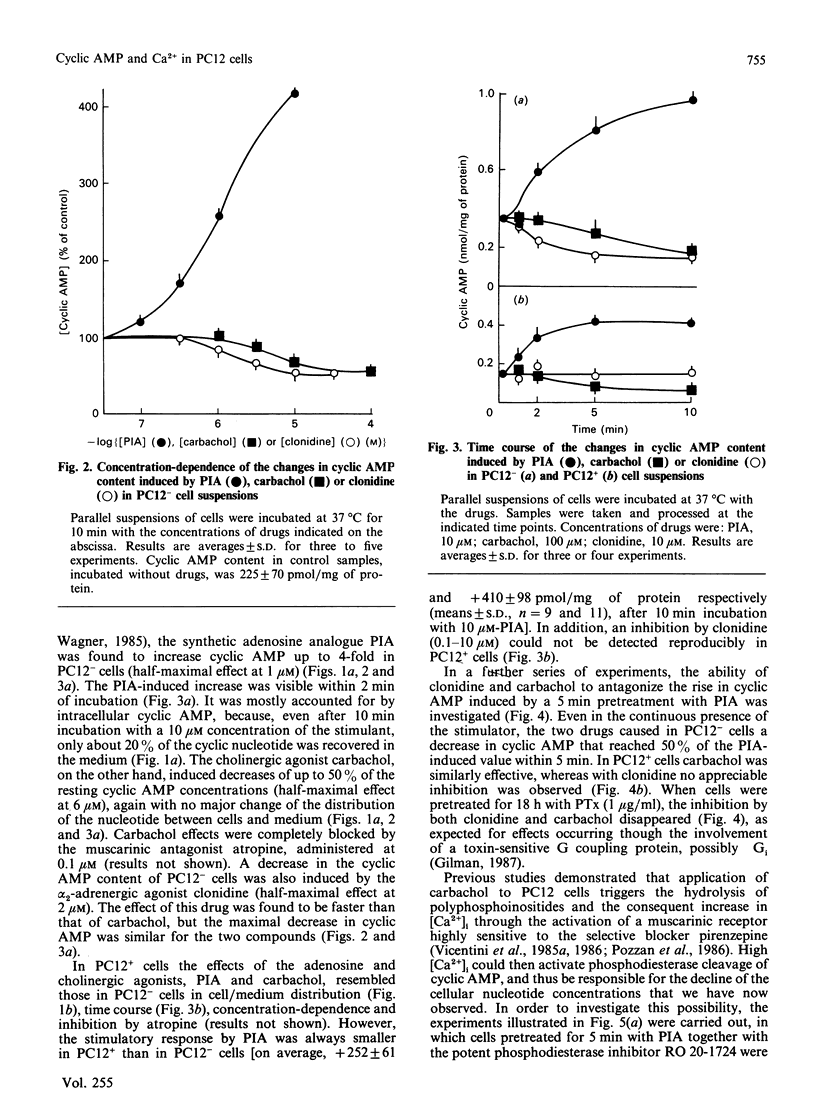
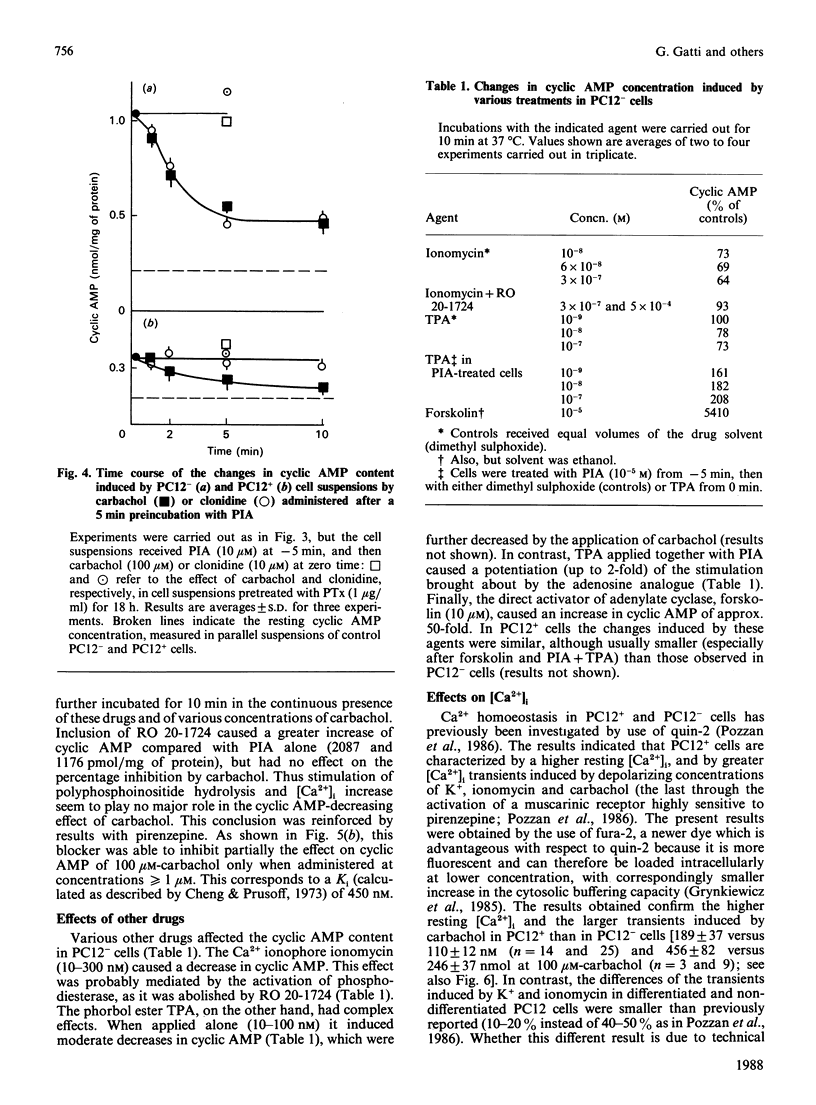
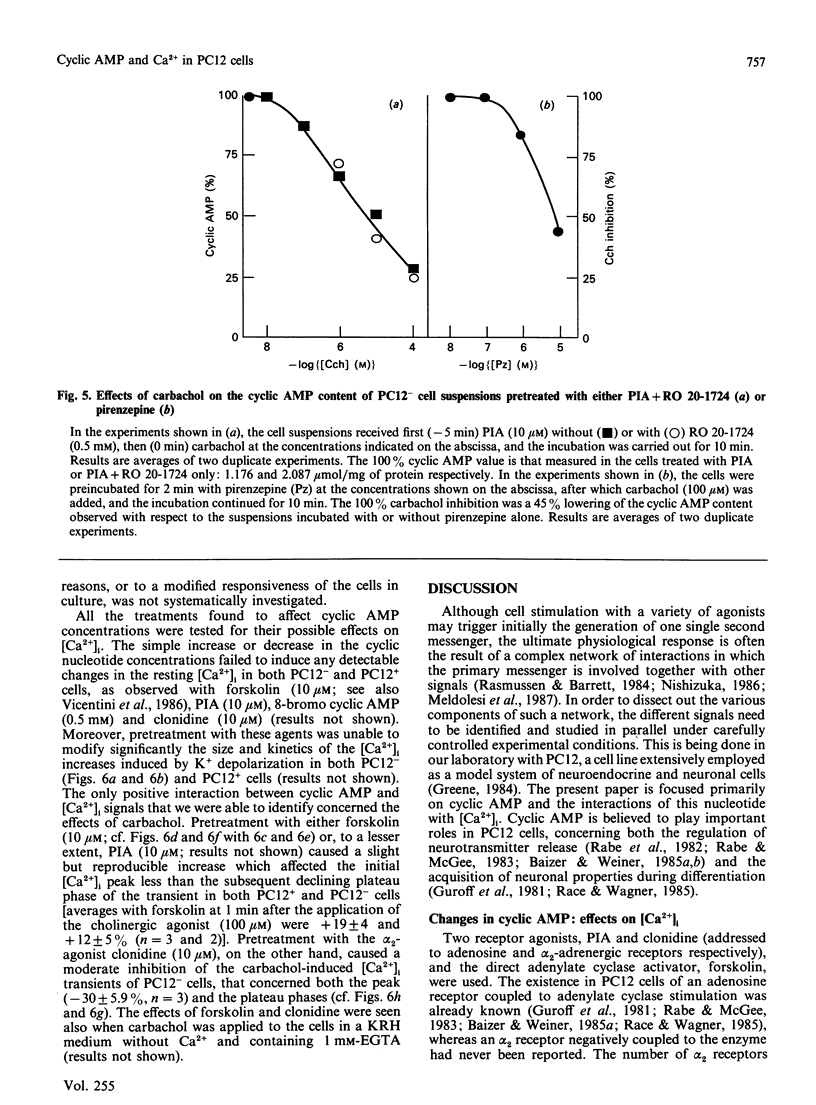
Changes in cyclic AMP concentrations were studied in intact PC12 pheochromocytoma cells exposed to a variety of treatments. A marked increase was triggered by N-(L-2-phenylisopropyl)adenosine, the activator of an adenosine receptor, whereas a decrease (observed even after phosphodiesterase blockade) was induced by carbachol, working through a muscarinic receptor inhibited by the selective muscarinic blocker pirenzepine, only at high concentration (Ki 450 nM). A decrease in cyclic AMP was also induced by clonidine, an alpha 2-adrenergic-receptor agonist. Both the alpha 2-adrenergic and the muscarinic inhibitions were prevented by pretreatment of the cells with pertussis toxin, and were unaffected by the phorbol ester 12-O-tetradecanoylphorbol 13-acetate. The latter drug caused a decrease in the resting cyclic AMP concentrations, and a potentiation of the increase induced by adenosine-receptor activation. Except for clonidine, all these treatments were found to be effective in both growing PC12 cells and, although to a smaller degree, in cells that had stopped growing and had acquired a neuron-like phenotype after prolonged treatment with nerve growth factor (NGF). Neither forskolin (a direct activator of adenylate cyclase) nor the activation of adenosine and alpha-adrenergic receptors was able to modify the resting cytosolic Ca2+ concentration [Ca2+]i in PC12 cells. Likewise, the K+-induced [Ca2+]i transients were unchanged after these treatments, whereas the transients induced by carbachol through the activation of a muscarinic receptor highly sensitive to pirenzepine were moderately potentiated by forskolin (and, to a lesser degree, by the adenosine analogue) and attenuated by clonidine. These results characterize in further detail the spectrum and the mutual interrelationships of the intracellular signals induced by receptor activation in PC12 cells, also as a function of the NGF-induced differentiation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baizer L., Weiner N. Nerve growth factor treatment enhances nicotine-stimulated dopamine release and increases in cyclic adenosine 3':5'-monophosphate levels in PC12 cell cultures. J Neurosci. 1985 May;5(5):1176–1179. doi: 10.1523/JNEUROSCI.05-05-01176.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baizer L., Weiner N. Regulation of dopamine release from PC12 pheochromocytoma cell cultures during stimulation with elevated potassium or carbachol. J Neurochem. 1985 Feb;44(2):495–501. doi: 10.1111/j.1471-4159.1985.tb05441.x. [DOI] [PubMed] [Google Scholar]
- Bouvier M., Leeb-Lundberg L. M., Benovic J. L., Caron M. G., Lefkowitz R. J. Regulation of adrenergic receptor function by phosphorylation. II. Effects of agonist occupancy on phosphorylation of alpha 1- and beta 2-adrenergic receptors by protein kinase C and the cyclic AMP-dependent protein kinase. J Biol Chem. 1987 Mar 5;262(7):3106–3113. [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F., Pozzan T., Wollheim C. B., Vicentini L. M., Meldolesi J. Tumor promoter phorbol myristate acetate inhibits Ca2+ influx through voltage-gated Ca2+ channels in two secretory cell lines, PC12 and RINm5F. J Biol Chem. 1986 Jan 5;261(1):32–35. [PubMed] [Google Scholar]
- Dragunow M., Robertson H. A. Seizure-inducible c-fos protein(s) in mammalian neurons. Trends Pharmacol Sci. 1988 Jan;9(1):5–6. doi: 10.1016/0165-6147(88)90229-5. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene S. L., Perry H. O. Is it skin cancer--or keratoacanthoma? Geriatrics. 1984 Sep;39(9):91-4, 98-9, 102. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Guroff G., Dickens G., End D., Londos C. The action of adenosine analogs on PC12 cells. J Neurochem. 1981 Dec;37(6):1431–1439. doi: 10.1111/j.1471-4159.1981.tb06312.x. [DOI] [PubMed] [Google Scholar]
- Katada T., Gilman A. G., Watanabe Y., Bauer S., Jakobs K. H. Protein kinase C phosphorylates the inhibitory guanine-nucleotide-binding regulatory component and apparently suppresses its function in hormonal inhibition of adenylate cyclase. Eur J Biochem. 1985 Sep 2;151(2):431–437. doi: 10.1111/j.1432-1033.1985.tb09120.x. [DOI] [PubMed] [Google Scholar]
- Malgaroli A., Milani D., Meldolesi J., Pozzan T. Fura-2 measurement of cytosolic free Ca2+ in monolayers and suspensions of various types of animal cells. J Cell Biol. 1987 Nov;105(5):2145–2155. doi: 10.1083/jcb.105.5.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldolesi J., Gatti G., Ambrosini A., Pozzan T., Westhead E. W. Second-messenger control of catecholamine release from PC12 cells. Role of muscarinic receptors and nerve-growth-factor-induced cell differentiation. Biochem J. 1988 Nov 1;255(3):761–768. doi: 10.1042/bj2550761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Pierce J., Suelter C. H. An evaluation of the Coomassie brillant blue G-250 dye-binding method for quantitative protein determination. Anal Biochem. 1977 Aug;81(2):478–480. doi: 10.1016/0003-2697(77)90723-0. [DOI] [PubMed] [Google Scholar]
- Pozzan T., Di Virgilio F., Vicentini L. M., Meldolesi J. Activation of muscarinic receptors in PC12 cells. Stimulation of Ca2+ influx and redistribution. Biochem J. 1986 Mar 15;234(3):547–553. doi: 10.1042/bj2340547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzan T., Gatti G., Dozio N., Vicentini L. M., Meldolesi J. Ca2+-dependent and -independent release of neurotransmitters from PC12 cells: a role for protein kinase C activation? J Cell Biol. 1984 Aug;99(2):628–638. doi: 10.1083/jcb.99.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabe C. S., McGee R., Jr Regulation of depolarization-dependent release of neurotransmitters by adenosine: cyclic AMP-dependent enhancement of release from PC12 cells. J Neurochem. 1983 Dec;41(6):1623–1634. doi: 10.1111/j.1471-4159.1983.tb00873.x. [DOI] [PubMed] [Google Scholar]
- Rabe C. S., Schneider J., McGee R., Jr Enhancement of depolarization-dependent neurosecretion from PC12 cells by forskolin-induced elevation of cyclic AMP. J Cyclic Nucleotide Res. 1982;8(6):371–384. [PubMed] [Google Scholar]
- Race H. M., Wagner J. A. Nerve growth factor affects cyclic AMP metabolism, but not by directly stimulating adenylate cyclase activity. J Neurochem. 1985 May;44(5):1588–1592. doi: 10.1111/j.1471-4159.1985.tb08799.x. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Barrett P. Q. Calcium messenger system: an integrated view. Physiol Rev. 1984 Jul;64(3):938–984. doi: 10.1152/physrev.1984.64.3.938. [DOI] [PubMed] [Google Scholar]
- Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983 Feb 17;301(5901):569–574. doi: 10.1038/301569a0. [DOI] [PubMed] [Google Scholar]
- Vicentini L. M., Ambrosini A., Di Virgilio F., Meldolesi J., Pozzan T. Activation of muscarinic receptors in PC12 cells. Correlation between cytosolic Ca2+ rise and phosphoinositide hydrolysis. Biochem J. 1986 Mar 15;234(3):555–562. doi: 10.1042/bj2340555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicentini L. M., Ambrosini A., Di Virgilio F., Pozzan T., Meldolesi J. Muscarinic receptor-induced phosphoinositide hydrolysis at resting cytosolic Ca2+ concentration in PC12 cells. J Cell Biol. 1985 Apr;100(4):1330–1333. doi: 10.1083/jcb.100.4.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicentini L. M., Di Virgilio F., Ambrosini A., Pozzan T., Meldolesi J. Tumor promoter phorbol 12-myristate, 13-acetate inhibits phosphoinositide hydrolysis and cytosolic Ca2+ rise induced by the activation of muscarinic receptors in PC12 cells. Biochem Biophys Res Commun. 1985 Feb 28;127(1):310–317. doi: 10.1016/s0006-291x(85)80160-1. [DOI] [PubMed] [Google Scholar]
- Yoshimasa T., Sibley D. R., Bouvier M., Lefkowitz R. J., Caron M. G. Cross-talk between cellular signalling pathways suggested by phorbol-ester-induced adenylate cyclase phosphorylation. Nature. 1987 May 7;327(6117):67–70. doi: 10.1038/327067a0. [DOI] [PubMed] [Google Scholar]


