Abstract
ATPase activities were measured in surface membranes and mitochondria isolated from promastigotes of the parasitic protozoan Leishmania donovani. The two enzymes were differentiated on the basis of pH optima, inhibitor sensitivity and by immunochemical methods. The surface-membrane (SM-) ATPase had an activity of 100 nmol/min per mg of protein, which was optimal at pH 6.5. The enzyme was Mg2+-dependent, partially inhibited by Ca2+, and unaffected by Na+ or K+. The SM-ATPase was inhibited by orthovanadate, NN'-dicyclohexylcarbodi-imide, and N-ethylmaleimide [IC50 (concentration causing half-maximal inhibition) 7.5, 25 and 520 microM respectively]; however, it was unaffected by ouabain, azide or oligomycin. The SM-ATPase demonstrated a Km of 1.05 mM and a Vmax. of 225 nmol/min per mg of protein. Moreover, fine-structure cytochemical results demonstrated that the SM-ATPase was localized to the cytoplasmic lamina of the parasite SM. A method was devised for the isolation of SM-derived vesicles. These were used to demonstrate the proton-pumping capacity of the SM-ATPase. Cumulatively, these results constitute the first demonstration of a surface-membrane proton-translocating ATPase in a parasitic protozoan.
Full text
PDF


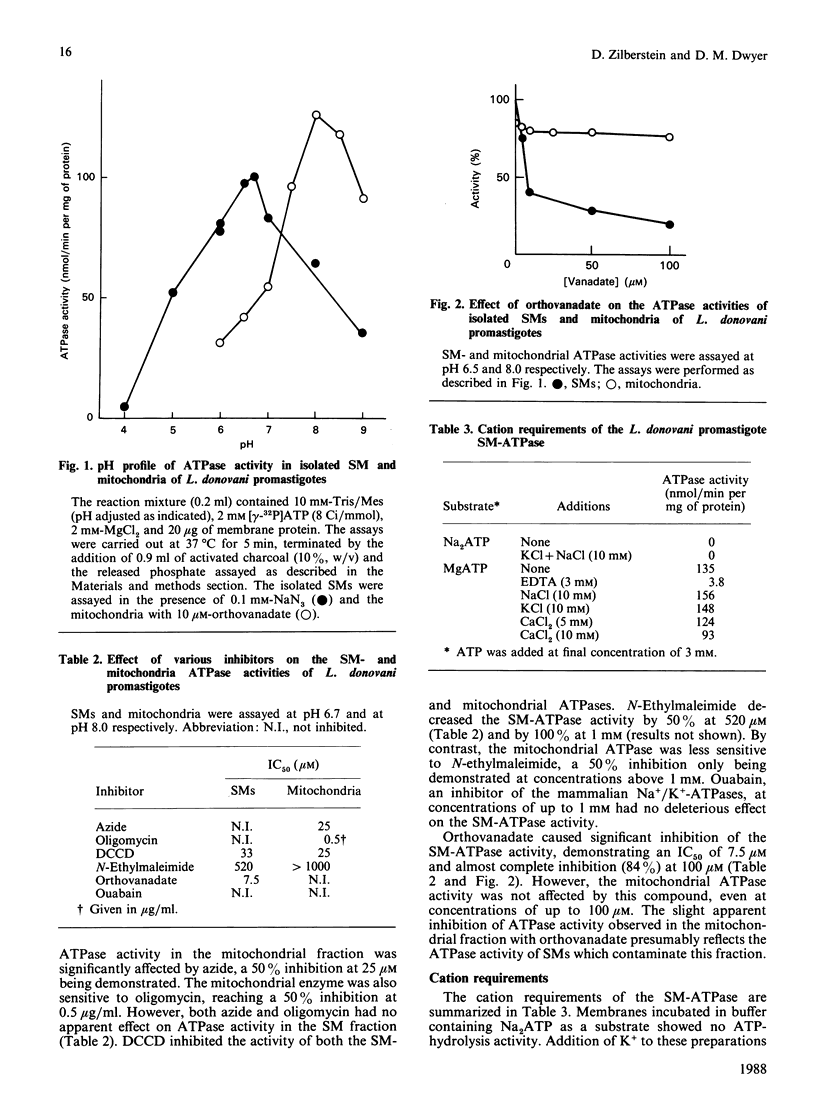
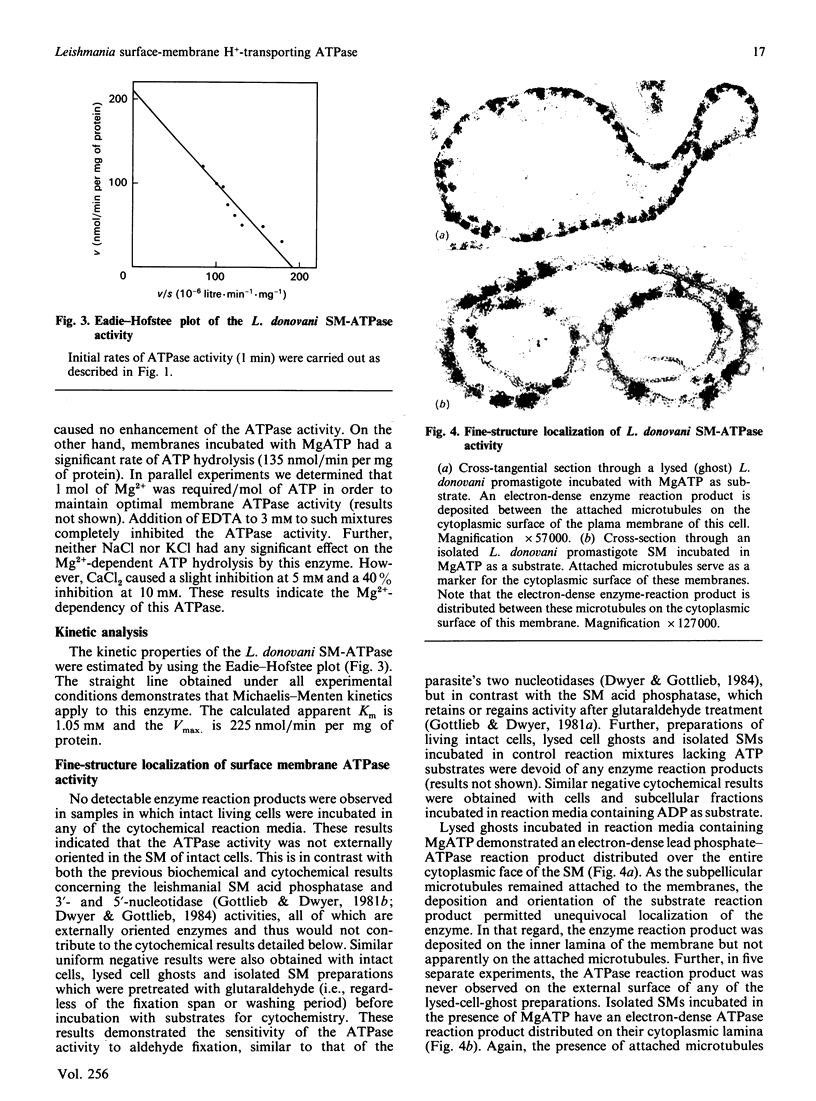

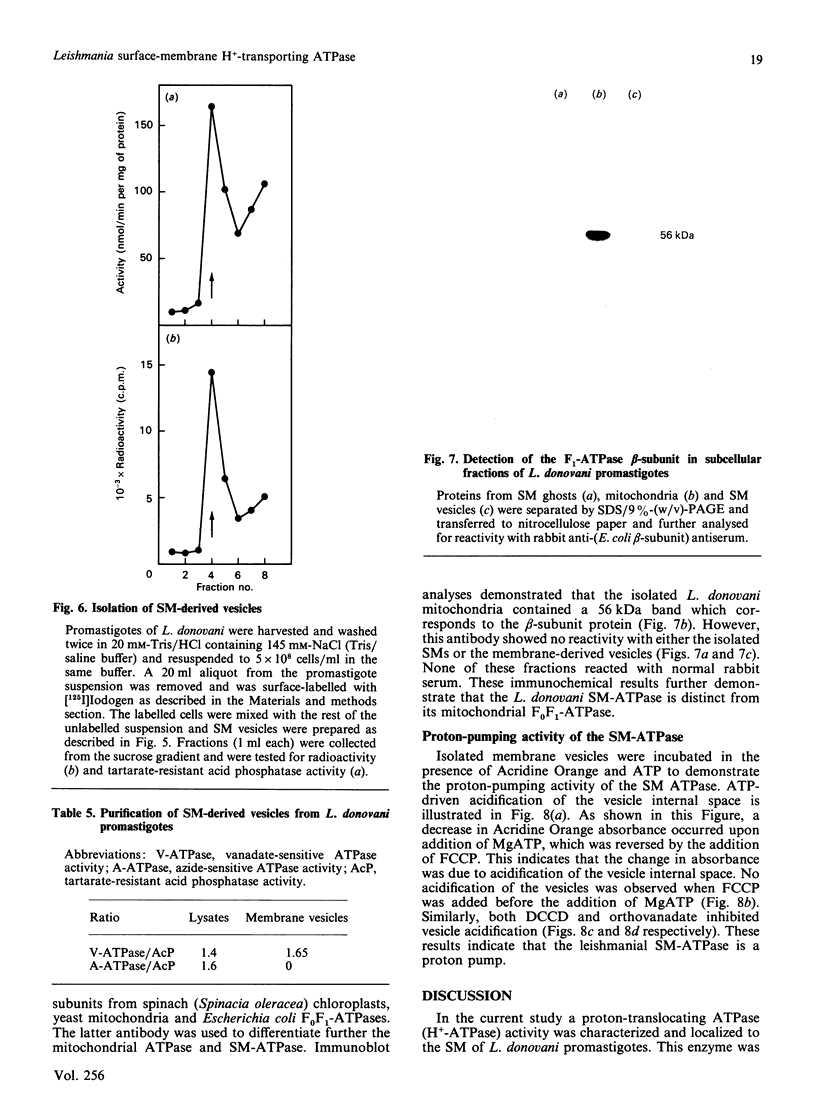

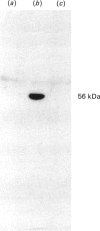
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Awqati Q. Proton-translocating ATPases. Annu Rev Cell Biol. 1986;2:179–199. doi: 10.1146/annurev.cb.02.110186.001143. [DOI] [PubMed] [Google Scholar]
- Berman J. D., Dwyer D. M., Wyler D. J. Multiplication of Leishmania in human macrophages in vitro. Infect Immun. 1979 Oct;26(1):375–379. doi: 10.1128/iai.26.1.375-379.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H., Farquhar M. G. Presence of adenylate cyclase activity in Golgi and other fractions from rat liver. II. Cytochemical localization within Golgi and ER membranes. J Cell Biol. 1976 Sep;70(3):671–684. doi: 10.1083/jcb.70.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cidon S., Ben-David H., Nelson N. ATP-driven proton fluxes across membranes of secretory organelles. J Biol Chem. 1983 Oct 10;258(19):11684–11688. [PubMed] [Google Scholar]
- Dupont F. M., Bennett A. B., Spanswick R. M. Localization of a proton-translocating ATPase on sucrose gradients. Plant Physiol. 1982 Oct;70(4):1115–1119. doi: 10.1104/pp.70.4.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer D. M., Gottlieb M. Surface membrane localization of 3'- and 5'-nucleotidase activities in Leishmania donovani promastigotes. Mol Biochem Parasitol. 1984 Feb;10(2):139–150. doi: 10.1016/0166-6851(84)90002-1. [DOI] [PubMed] [Google Scholar]
- Dwyer D. M., Gottlieb M. The surface membrane chemistry of Leishmania: its possible role in parasite sequestration and survival. J Cell Biochem. 1983;23(1-4):35–45. doi: 10.1002/jcb.240230105. [DOI] [PubMed] [Google Scholar]
- Dwyer D. M. Isolation and partial characterization of surface membranes from Leishmania donovani promastigotes. J Protozool. 1980 May;27(2):176–182. doi: 10.1111/j.1550-7408.1980.tb04676.x. [DOI] [PubMed] [Google Scholar]
- Dwyer D. M. Leishmania donovani: surface membrane carbohydrates of promastigotes. Exp Parasitol. 1977 Apr;41(2):341–358. doi: 10.1016/0014-4894(77)90107-2. [DOI] [PubMed] [Google Scholar]
- Eddy A. A., Indge K. J., Backen K., Nowacki J. A. Interctions between potassium ions and glycine transport in the yeast Saccharomyces carlsbergensis. Biochem J. 1970 Dec;120(4):845–852. doi: 10.1042/bj1200845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar M. G., Bergeron J. J., Palade G. E. Cytochemistry of Golgi fractions prepared from rat liver. J Cell Biol. 1974 Jan;60(1):8–25. doi: 10.1083/jcb.60.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishkes H., Rudnick G. Bioenergetics of serotonin transport by membrane vesicles derived from platelet dense granules. J Biol Chem. 1982 May 25;257(10):5671–5677. [PubMed] [Google Scholar]
- Galloway C. J., Dean G. E., Marsh M., Rudnick G., Mellman I. Acidification of macrophage and fibroblast endocytic vesicles in vitro. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3334–3338. doi: 10.1073/pnas.80.11.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner P. R., Dwyer D. M. Radioiodination and identification of externally disposed membrane components of Leishmania tropica. Mol Biochem Parasitol. 1983 Aug;8(4):283–295. doi: 10.1016/0166-6851(83)90075-0. [DOI] [PubMed] [Google Scholar]
- Goffeau A., Slayman C. W. The proton-translocating ATPase of the fungal plasma membrane. Biochim Biophys Acta. 1981 Dec 30;639(3-4):197–223. doi: 10.1016/0304-4173(81)90010-0. [DOI] [PubMed] [Google Scholar]
- Gottlieb M., Dwyer D. M. Leishmania donovani: surface membrane acid phosphatase activity of promastigotes. Exp Parasitol. 1981 Aug;52(1):117–128. doi: 10.1016/0014-4894(81)90067-9. [DOI] [PubMed] [Google Scholar]
- Kaback H. R. The lac carrier protein in Escherichia coli. J Membr Biol. 1983;76(2):95–112. doi: 10.1007/BF02000610. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T., Palade G. E. The localization of Mg-Na-K-activated adenosine triphosphatase on red cell ghost membranes. J Cell Biol. 1967 Nov;35(2):385–404. doi: 10.1083/jcb.35.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J. A high affinity Ca2+-dependent ATPase in the surface membrane of the bloodstream stage of Trypanosoma rhodesiense. Mol Biochem Parasitol. 1985 May;15(2):189–201. doi: 10.1016/0166-6851(85)90119-7. [DOI] [PubMed] [Google Scholar]
- Padan E., Schuldiner S. Intracellular pH and membrane potential as regulators in the prokaryotic cell. J Membr Biol. 1987;95(3):189–198. doi: 10.1007/BF01869481. [DOI] [PubMed] [Google Scholar]
- Padan E., Zilberstein D., Schuldiner S. pH homeostasis in bacteria. Biochim Biophys Acta. 1981 Dec;650(2-3):151–166. doi: 10.1016/0304-4157(81)90004-6. [DOI] [PubMed] [Google Scholar]
- Perlin D. S., Kasamo K., Brooker R. J., Slayman C. W. Electrogenic H+ translocation by the plasma membrane ATPase of Neurospora. Studies on plasma membrane vesicles and reconstituted enzyme. J Biol Chem. 1984 Jun 25;259(12):7884–7892. [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Serrano R. Plasma membrane ATPase of fungi and plants as a novel type of proton pump. Curr Top Cell Regul. 1984;23:87–126. doi: 10.1016/b978-0-12-152823-2.50007-6. [DOI] [PubMed] [Google Scholar]
- Timm S. L., Leon L. L., Pereira N. M., de Souza W., Queiroz-Cruz M., Bräscher H. M., Lima A. O. Isolation of an enriched plasma membrane-subpellicular microtubule fraction of Leishmania mexicana amazonensis. Mem Inst Oswaldo Cruz. 1980;75(1-2):145–155. doi: 10.1590/s0074-02761980000100014. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. C., Kreiner P., Barrnett R. J., Bitensky M. W. Biochemical characterization and cytochemical localization of a catecholamine-sensitive adenylate cyclase in isolated capillary endothelium. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3175–3179. doi: 10.1073/pnas.69.11.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widnell C. C. Cytochemical localization of 5'-nucleotidase in subcellular fractions isolated from rat liver. I. The origin of 5'-nucleotidase activity in microsomes. J Cell Biol. 1972 Mar;52(3):542–558. doi: 10.1083/jcb.52.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberstein D., Dwyer D. M. Antidepressants cause lethal disruption of membrane function in the human protozoan parasite Leishmania. Science. 1984 Nov 23;226(4677):977–979. doi: 10.1126/science.6505677. [DOI] [PubMed] [Google Scholar]
- Zilberstein D., Dwyer D. M., Matthaei S., Horuk R. Identification and biochemical characterization of the plasma membrane glucose transporter of Leishmania donovani. J Biol Chem. 1986 Nov 15;261(32):15053–15057. [PubMed] [Google Scholar]
- Zilberstein D., Dwyer D. M. Protonmotive force-driven active transport of D-glucose and L-proline in the protozoan parasite Leishmania donovani. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1716–1720. doi: 10.1073/pnas.82.6.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberstein D., Schuldiner S., Padan E. Proton electrochemical gradient in Escherichia coli cells and its relation to active transport of lactose. Biochemistry. 1979 Feb 20;18(4):669–673. doi: 10.1021/bi00571a018. [DOI] [PubMed] [Google Scholar]
- Zilberstein D., Shirvan M. H., Barile M. F., Rottem S. The beta-subunit of the F1F0-ATPase is conserved in mycoplasmas. J Biol Chem. 1986 Jun 5;261(16):7109–7111. [PubMed] [Google Scholar]