Abstract
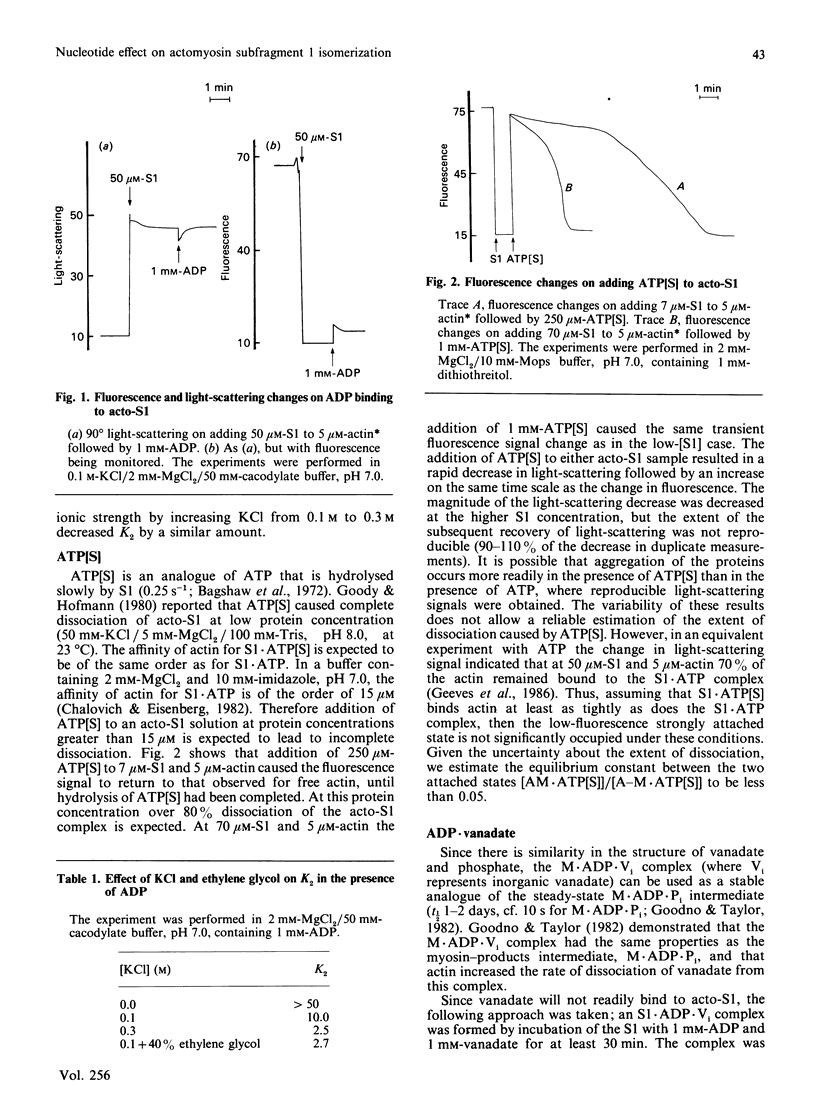
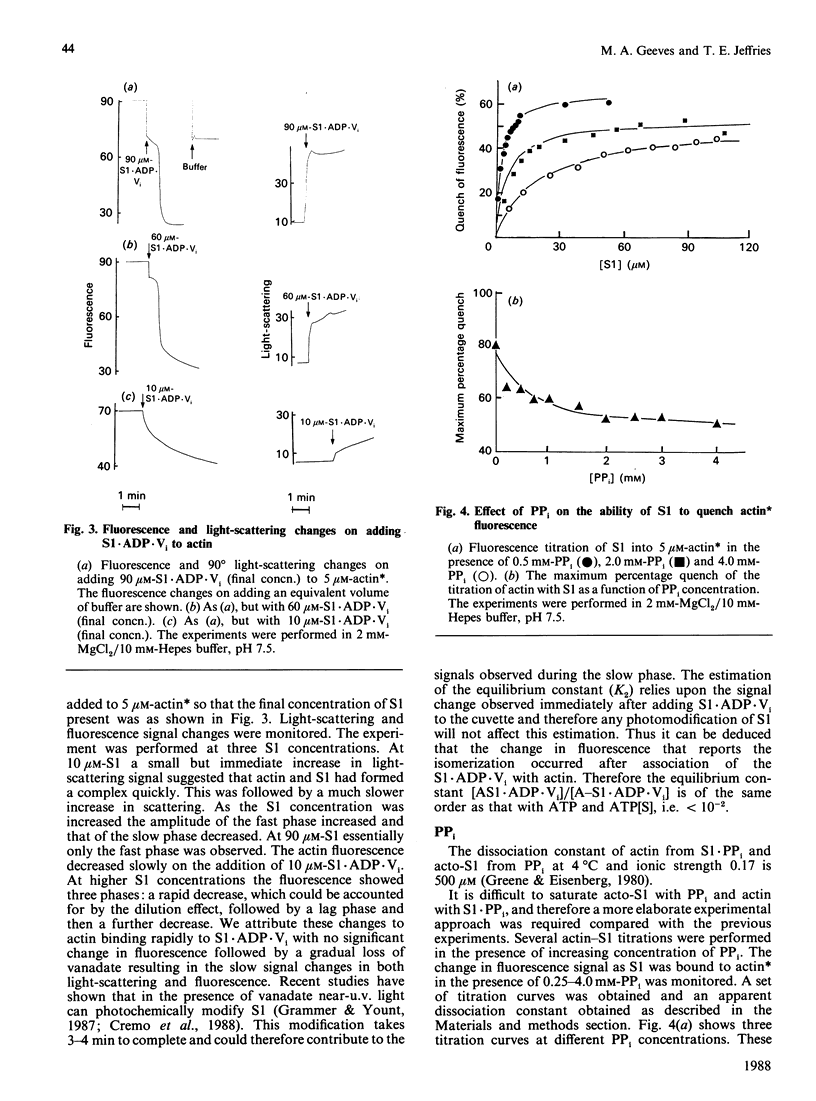
The binding of actin to myosin subfragment 1 (S1) has been shown to occur as a two-step reaction [Coates, Criddle & Geeves (1985) Biochem. J. 232, 351-356]. In the first step actin is weakly bound and the second step involves the complex isomerizing to a more tightly bound state. This isomerization can be followed specifically by monitoring the fluorescence of actin that has been covalently labelled with N-(pyren-1-yl)-iodoacetamide at Cys-374 [Geeves, Jeffries & Millar (1986) Biochemistry 25, 8454-8458]. We report here that the presence of nucleotides and nucleotide analogues affects the equilibrium between the strongly bound and weakly bound states (referred to as K2). In the presence of ATP, [gamma-thio]ATP or ADP and vanadate a value of approx. less than 10(-2) was estimated for K2. In the presence of PPi or ADP a value of approx. 2.3 or 10 respectively was obtained. An increase in KCl concentration or the presence of 40% ethylene glycol was found to decrease K2 in the presence of ADP. The data presented here are consistent with the two-step binding model proposed by Geeves, Goody & Gutfreund [(1984) J. Muscle Res. Cell Motil. 5, 351-361], where it was suggested that the transition between weakly bound and strongly bound states is closely associated with the force-generating event in whole muscle.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Botts J., Takashi R., Torgerson P., Hozumi T., Muhlrad A., Mornet D., Morales M. F. On the mechanism of energy transduction in myosin subfragment 1. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2060–2064. doi: 10.1073/pnas.81.7.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalovich J. M., Eisenberg E. Inhibition of actomyosin ATPase activity by troponin-tropomyosin without blocking the binding of myosin to actin. J Biol Chem. 1982 Mar 10;257(5):2432–2437. [PMC free article] [PubMed] [Google Scholar]
- Coates J. H., Criddle A. H., Geeves M. A. Pressure-relaxation studies of pyrene-labelled actin and myosin subfragment 1 from rabbit skeletal muscle. Evidence for two states of acto-subfragment 1. Biochem J. 1985 Dec 1;232(2):351–356. doi: 10.1042/bj2320351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criddle A. H., Geeves M. A., Jeffries T. The use of actin labelled with N-(1-pyrenyl)iodoacetamide to study the interaction of actin with myosin subfragments and troponin/tropomyosin. Biochem J. 1985 Dec 1;232(2):343–349. doi: 10.1042/bj2320343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg E., Greene L. E. The relation of muscle biochemistry to muscle physiology. Annu Rev Physiol. 1980;42:293–309. doi: 10.1146/annurev.ph.42.030180.001453. [DOI] [PubMed] [Google Scholar]
- Geeves M. A., Goody R. S., Gutfreund H. Kinetics of acto-S1 interaction as a guide to a model for the crossbridge cycle. J Muscle Res Cell Motil. 1984 Aug;5(4):351–361. doi: 10.1007/BF00818255. [DOI] [PubMed] [Google Scholar]
- Geeves M. A., Gutfreund H. The use of pressure perturbations to investigate the interaction of rabbit muscle myosin subfragment 1 with actin in the presence of MgADP. FEBS Lett. 1982 Apr 5;140(1):11–15. doi: 10.1016/0014-5793(82)80509-7. [DOI] [PubMed] [Google Scholar]
- Geeves M. A., Jeffries T. E., Millar N. C. ATP-induced dissociation of rabbit skeletal actomyosin subfragment 1. Characterization of an isomerization of the ternary acto-S1-ATP complex. Biochemistry. 1986 Dec 30;25(26):8454–8458. doi: 10.1021/bi00374a020. [DOI] [PubMed] [Google Scholar]
- Goodno C. C., Taylor E. W. Inhibition of actomyosin ATPase by vanadate. Proc Natl Acad Sci U S A. 1982 Jan;79(1):21–25. doi: 10.1073/pnas.79.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goody R. S., Hofmann W. Stereochemical aspects of the interaction of myosin and actomyosin with nucleotides. J Muscle Res Cell Motil. 1980 Mar;1(1):101–115. doi: 10.1007/BF00711928. [DOI] [PubMed] [Google Scholar]
- Greene L. E., Eisenberg E. Dissociation of the actin.subfragment 1 complex by adenyl-5'-yl imidodiphosphate, ADP, and PPi. J Biol Chem. 1980 Jan 25;255(2):543–548. [PubMed] [Google Scholar]
- Kouyama T., Mihashi K. Fluorimetry study of N-(1-pyrenyl)iodoacetamide-labelled F-actin. Local structural change of actin protomer both on polymerization and on binding of heavy meromyosin. Eur J Biochem. 1981;114(1):33–38. [PubMed] [Google Scholar]
- Lehrer S. S., Kerwar G. Intrinsic fluorescence of actin. Biochemistry. 1972 Mar 28;11(7):1211–1217. doi: 10.1021/bi00757a015. [DOI] [PubMed] [Google Scholar]
- Margossian S. S., Lowey S. Interaction of myosin subfragments with F-actin. Biochemistry. 1978 Dec 12;17(25):5431–5439. doi: 10.1021/bi00618a017. [DOI] [PubMed] [Google Scholar]
- Shriver J. W., Sykes B. D. Phosphorus-31 nuclear magnetic resonance evidence for two conformations of myosin subfragment-1.nucleotide complexes. Biochemistry. 1981 Mar 31;20(7):2004–2012. doi: 10.1021/bi00510a041. [DOI] [PubMed] [Google Scholar]
- Trybus K. M., Taylor E. W. Transient kinetics of adenosine 5'-diphosphate and adenosine 5'-(beta, gamma-imidotriphosphate) binding to subfragment 1 and actosubfragment 1. Biochemistry. 1982 Mar 16;21(6):1284–1294. doi: 10.1021/bi00535a028. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Taylor R. S. Separation of subfragment-1 isoenzymes from rabbit skeletal muscle myosin. Nature. 1975 Sep 4;257(5521):54–56. doi: 10.1038/257054a0. [DOI] [PubMed] [Google Scholar]
- West J. J., Nagy B., Gergely J. The effect of EDTA on spectral properties of ATP-, ADP-, and ITP-G-actin. Biochem Biophys Res Commun. 1967 Nov 30;29(4):611–616. doi: 10.1016/0006-291x(67)90530-x. [DOI] [PubMed] [Google Scholar]


