Abstract
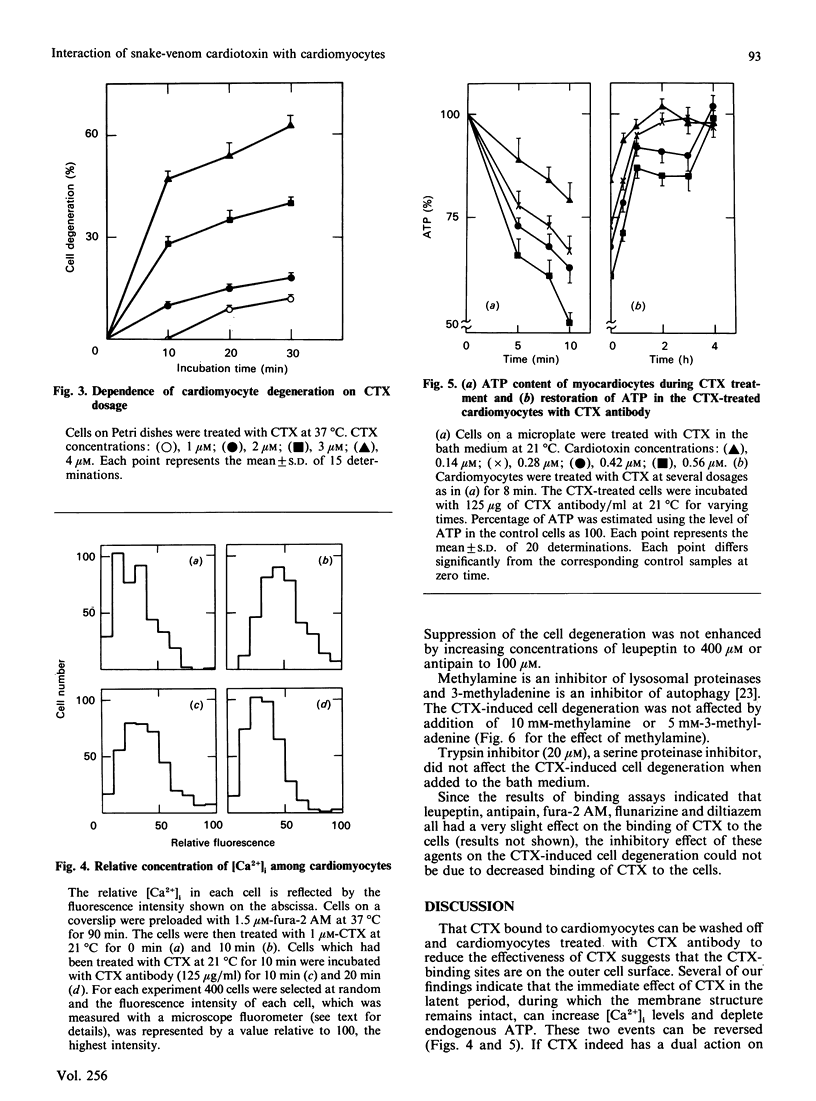
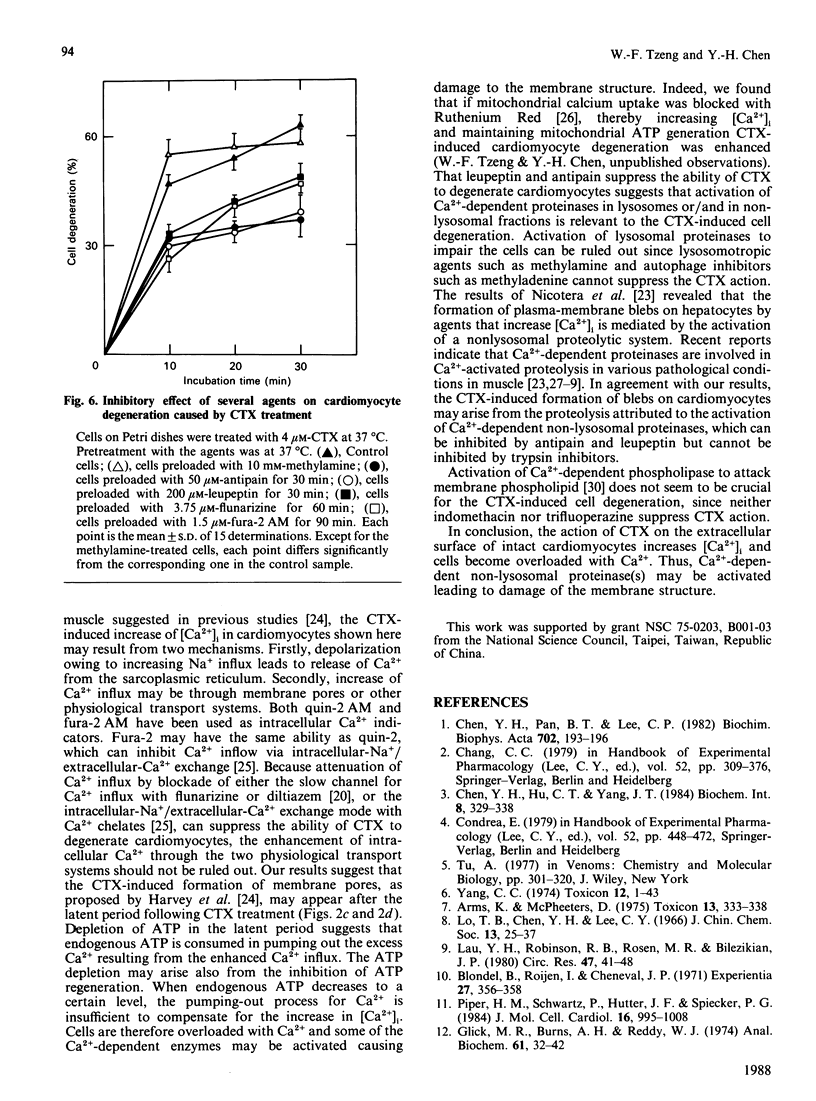
The incubation of 10(5) single neonatal rat cardiomyocytes with 1 microM-cardiotoxin in a bath medium, Tyrode solution in the presence of 1 mM-Ca2+, at 37 degrees C evoked the following chain of events. Firstly, there appeared a latent period of about 10 min during which the cells behaved normally. Neither lactate dehydrogenase nor ATP leaked from the cells. Cytosolic free Ca2+ increased considerably, as measured by the fluorescence intensity of fura-2-Ca2+ complex. At the same time a large portion of endogenous ATP was depleted. Secondly, after the latent period, the cell beating became irregular and eventually stopped. Thirdly, blebs appeared on the cell surface, leading to cell degeneration. If, before the appearance of blebs, the cells were washed with the bath medium exhaustively or incubated in the presence of the toxin antibody, cytosolic free Ca2+ and endogenous ATP returned to normal levels and cells resumed regular beating. Preincubation of the cells with 3.75 microM-flunarizine or 3.75 microM-diltiazem (both are Ca2+ antagonists), or 1.5 microM-fura-2 acetoxymethyl ester (a chelate for Ca2+), or 200 microM-leupeptin or 50 microM-antipain (both are proteinase inhibitors) considerably suppressed the toxin's ability to degenerate the cells. On the other hand, lysosomal proteinase inhibitor, autophage inhibitor, serine proteinase inhibitor, phospholipase inhibitor and calmodulin antagonist did not inhibit the toxin's activity. The results suggest that the toxin may act on the extracellular surface of intact cardiomyocytes to increase cytosolic free Ca2+. The subsequent cell degeneration may result from the activation of a Ca2+-dependent non-lysosomal proteolytic system.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen T. J., Baker P. F. Intracellular Ca indicator Quin-2 inhibits Ca2+ inflow via Na/Ca exchange in squid axon. 1985 Jun 27-Jul 3Nature. 315(6022):755–756. doi: 10.1038/315755a0. [DOI] [PubMed] [Google Scholar]
- Arms K., McPheeters D. Sensitivity of cultured embryonic heart cells to cardiotoxin obtained from Naja naja siamensis venom. Toxicon. 1975 Nov;13(5):333–338. doi: 10.1016/0041-0101(75)90192-0. [DOI] [PubMed] [Google Scholar]
- Blondel B., Roijen I., Cheneval J. P. Heart cells in culture: a simple method for increasing the proportion of myoblasts. Experientia. 1971 Mar 15;27(3):356–358. doi: 10.1007/BF02138197. [DOI] [PubMed] [Google Scholar]
- Chen Y. H., Hu C. T., Yang J. T. Membrane disintegration and hemolysis of human erythrocytes by snake venom cardiotoxin (a membrane-disruptive polypeptide). Biochem Int. 1984 Feb;8(2):329–338. [PubMed] [Google Scholar]
- DeLuca M. A., Ingwall J. S., Bittl J. A. Biochemical responses of myocardial cells in culture to oxygen and glucose deprivation. Biochem Biophys Res Commun. 1974 Jul 24;59(2):749–756. doi: 10.1016/s0006-291x(74)80043-4. [DOI] [PubMed] [Google Scholar]
- Douillard J. Y., Hoffman T. Enzyme-linked immunosorbent assay for screening monoclonal antibody production using enzyme-labeled second antibody. Methods Enzymol. 1983;92:168–174. doi: 10.1016/0076-6879(83)92016-5. [DOI] [PubMed] [Google Scholar]
- Fox J. E., Phillips D. R. Role of phosphorylation in mediating the association of myosin with the cytoskeletal structures of human platelets. J Biol Chem. 1982 Apr 25;257(8):4120–4126. [PubMed] [Google Scholar]
- Glick M. R., Burns A. H., Reddy W. J. Dispersion and isolation of beating cells from adult rat heart. Anal Biochem. 1974 Sep;61(1):32–42. doi: 10.1016/0003-2697(74)90329-7. [DOI] [PubMed] [Google Scholar]
- Harvey A. L., Marshall R. J., Karlsson E. Effects of purified cardiotoxins from the Thailand cobra (Naja naja siamensis) on isolated skeletal and cardiac muscle preparations. Toxicon. 1982;20(2):379–396. doi: 10.1016/0041-0101(82)90001-0. [DOI] [PubMed] [Google Scholar]
- Higgins T. J., Allsopp D., Bailey P. J. The effect of extracellular calcium concentration and Ca-antagonist drugs on enzyme release and lactate production by anoxic heart cell cultures. J Mol Cell Cardiol. 1980 Sep;12(9):909–927. doi: 10.1016/0022-2828(80)90059-0. [DOI] [PubMed] [Google Scholar]
- Higgins T. J., Bailey P. J., Allsopp D. The influence of ATP depletion on the action of phospholipase C on cardiac myocyte membrane phospholipids. J Mol Cell Cardiol. 1981 Nov;13(11):1027–1030. doi: 10.1016/0022-2828(81)90478-8. [DOI] [PubMed] [Google Scholar]
- Hong C. Y., Chiang B. N., Ku J., Wei Y. H., Fong J. C. Calcium antagonists stimulate sperm motility in ejaculated human semen. Br J Clin Pharmacol. 1985 Jan;19(1):45–49. doi: 10.1111/j.1365-2125.1985.tb02611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesse R. L., Franson R. C. Modulation of purified phospholipase A2 activity from human platelets by calcium and indomethacin. Biochim Biophys Acta. 1979 Dec 18;575(3):467–470. doi: 10.1016/0005-2760(79)90117-6. [DOI] [PubMed] [Google Scholar]
- Kimes B. W., Brandt B. L. Characterization of two putative smooth muscle cell lines from rat thoracic aorta. Exp Cell Res. 1976 Mar 15;98(2):349–366. doi: 10.1016/0014-4827(76)90446-8. [DOI] [PubMed] [Google Scholar]
- Lau Y. H., Robinson R. B., Rosen M. R., Bilezikian J. P. Subclassification of beta-adrenergic receptors in cultured rat cardiac myoblasts and fibroblasts. Circ Res. 1980 Jul;47(1):41–48. doi: 10.1161/01.res.47.1.41. [DOI] [PubMed] [Google Scholar]
- LeJohn H. B., Stevenson R. M. D(-)-lactate dehydrogenases from fungi. Methods Enzymol. 1975;41:293–298. doi: 10.1016/s0076-6879(75)41067-9. [DOI] [PubMed] [Google Scholar]
- Libby P., Goldberg A. L. Leupeptin, a protease inhibitor, decreases protein degradation in normal and diseased muscles. Science. 1978 Feb 3;199(4328):534–536. doi: 10.1126/science.622552. [DOI] [PubMed] [Google Scholar]
- Nicotera P., Hartzell P., Baldi C., Svensson S. A., Bellomo G., Orrenius S. Cystamine induces toxicity in hepatocytes through the elevation of cytosolic Ca2+ and the stimulation of a nonlysosomal proteolytic system. J Biol Chem. 1986 Nov 5;261(31):14628–14635. [PubMed] [Google Scholar]
- Nicotera P., Hartzell P., Davis G., Orrenius S. The formation of plasma membrane blebs in hepatocytes exposed to agents that increase cytosolic Ca2+ is mediated by the activation of a non-lysosomal proteolytic system. FEBS Lett. 1986 Dec 1;209(1):139–144. doi: 10.1016/0014-5793(86)81099-7. [DOI] [PubMed] [Google Scholar]
- Piper H. M., Schwartz P., Hütter J. F., Spieckermann P. G. Energy metabolism and enzyme release of cultured adult rat heart muscle cells during anoxia. J Mol Cell Cardiol. 1984 Nov;16(11):995–1007. doi: 10.1016/s0022-2828(84)80013-9. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. A non-disruptive technique for loading calcium buffers and indicators into cells. Nature. 1981 Apr 9;290(5806):527–528. doi: 10.1038/290527a0. [DOI] [PubMed] [Google Scholar]
- Wier W. G., Cannell M. B., Berlin J. R., Marban E., Lederer W. J. Cellular and subcellular heterogeneity of [Ca2+]i in single heart cells revealed by fura-2. Science. 1987 Jan 16;235(4786):325–328. doi: 10.1126/science.3798114. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Williams R. J., Coll K. E., Thomas A. P. Cytosolic free Ca2+ concentration and intracellular calcium distribution of Ca2+-tolerant isolated heart cells. J Biol Chem. 1983 Nov 25;258(22):13411–13414. [PubMed] [Google Scholar]
- Yang C. C. Chemistry and evolution of toxins in snake venoms. Toxicon. 1974 Jan;12(1):1–43. doi: 10.1016/0041-0101(74)90096-8. [DOI] [PubMed] [Google Scholar]
- Zeman R. J., Kameyama T., Matsumoto K., Bernstein P., Etlinger J. D. Regulation of protein degradation in muscle by calcium. Evidence for enhanced nonlysosomal proteolysis associated with elevated cytosolic calcium. J Biol Chem. 1985 Nov 5;260(25):13619–13624. [PubMed] [Google Scholar]