Abstract
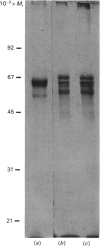
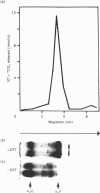
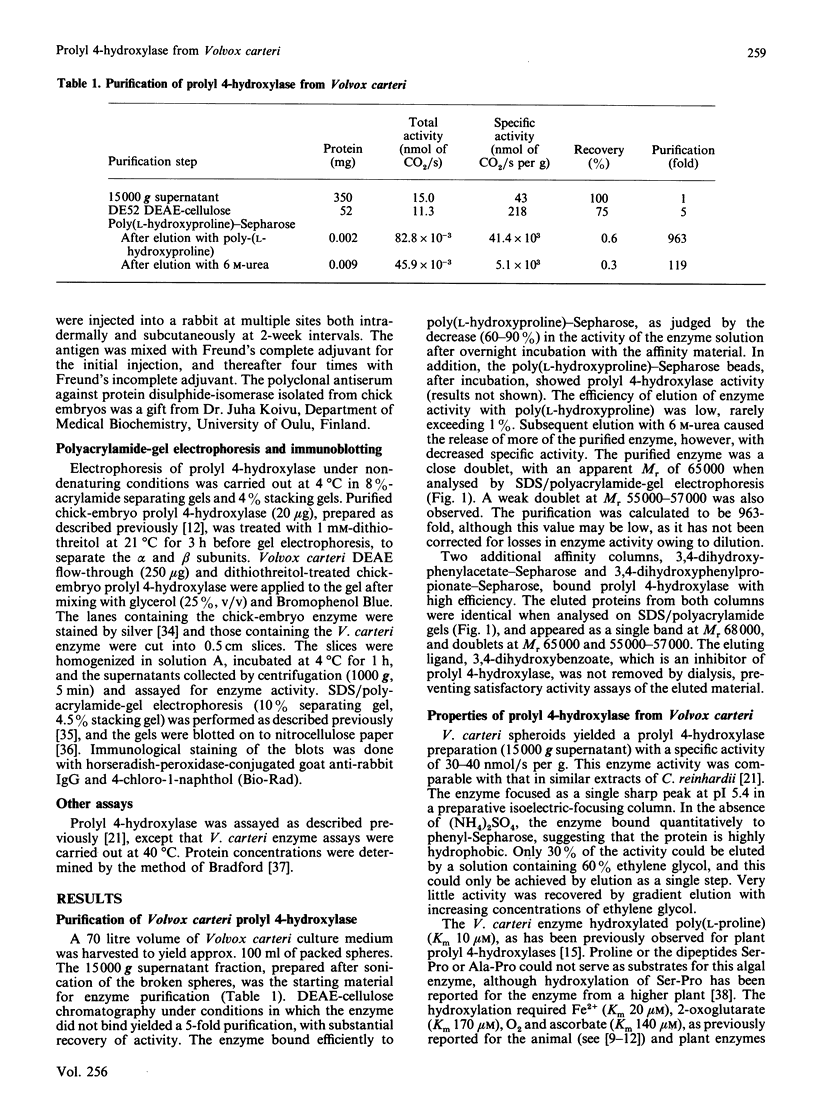
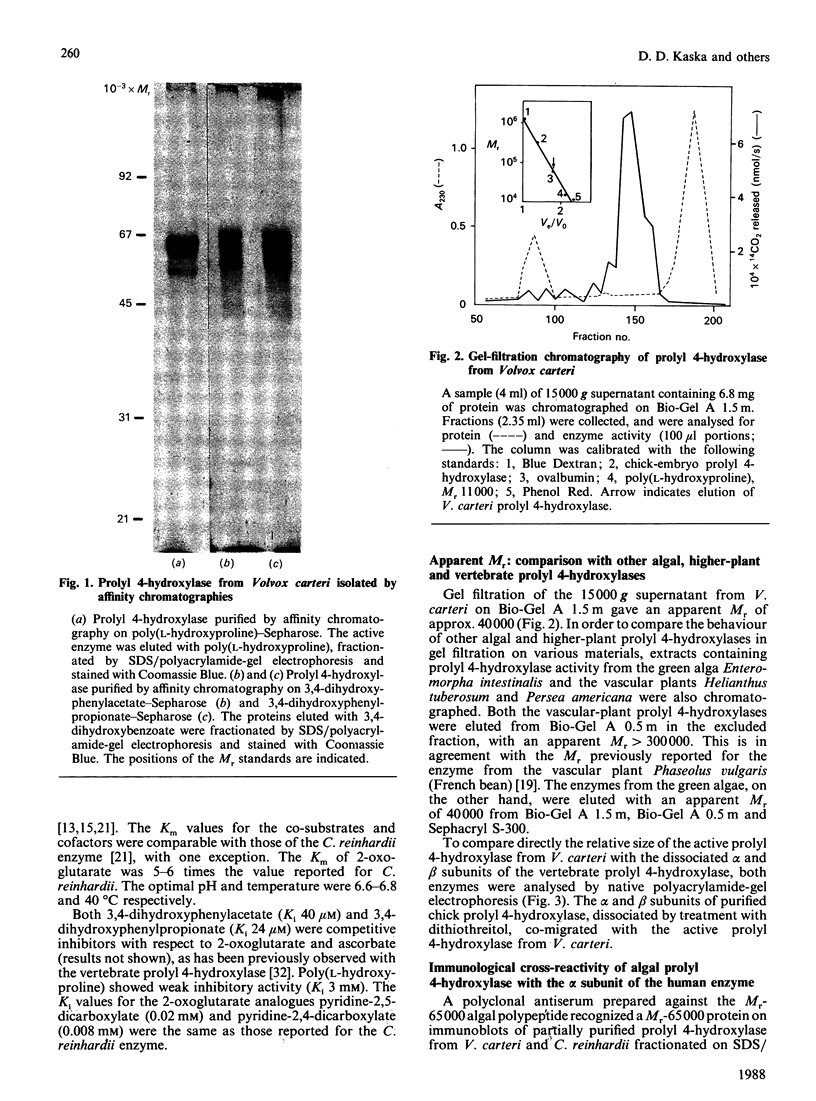
Prolyl 4-hydroxylase was isolated in a highly purified form from a multi-cellular green alga, Volvox carteri, by a procedure consisting of ion-exchange chromatography and affinity chromatography on poly(L-hydroxyproline) coupled to Sepharose. Two other affinity-column procedures were also developed, one involving 3,4-dihydroxyphenylacetate and the other 3,4-dihydroxyphenylpropionate linked to Sepharose. The Km values of the Volvox enzyme for the co-substrates and the peptide substrate, as well as the inhibition constants for selected 2-oxoglutarate analogues, were similar to those of the enzyme from Chlamydomonas reinhardii, except that the Km for 2-oxoglutarate with the Volvox enzyme was 6-fold greater. The temperature optimum of the Volvox enzyme was also 10 degrees C higher. The apparent Mr of the Volvox enzyme by gel filtration was about 40,000, being similar to that reported for the Chlamydomonas enzyme but markedly lower than that of the vertebrate enzymes. A similar apparent Mr of about 40,000 was also found for prolyl 4-hydroxylase from the green alga Enteromorpha intestinalis, whereas the enzyme from various vascular plants gave an apparent Mr greater than 300,000. SDS/polyacrylamide-gel electrophoresis demonstrated in the highly purified Volvox enzyme the presence of a major protein band doublet with a Mr of about 65,000 and a minor doublet of Mr about 55,000-57,000. A polyclonal antiserum, prepared against the Mr-65,000 doublet, stained in immunoblotting the Mr-65,000 doublet as well as the alpha subunit, but not the beta subunit, of the vertebrate prolyl 4-hydroxylase. An antiserum against the beta subunit of the vertebrate enzyme stained in immunoblotting a Mr-50,000 polypeptide in a partially purified Volvox enzyme preparation, but did not stain either the Mr-65,000 or the Mr-55,000-57,000 doublet of the highly purified enzyme. The data thus suggest that the active Volvox carteri prolyl 4-hydroxylase is an enzyme monomer antigenically related to the alpha subunit of the vertebrate enzyme.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolwell G. P., Robbins M. P., Dixon R. A. Elicitor-induced prolyl hydroxylase from French bean (Phaseolus vulgaris). Localization, purification and properties. Biochem J. 1985 Aug 1;229(3):693–699. doi: 10.1042/bj2290693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cassab G. I., Nieto-Sotelo J., Cooper J. B., van Holst G. J., Varner J. E. A developmentally regulated hydroxyproline-rich glycoprotein from the cell walls of soybean seed coats. Plant Physiol. 1985 Mar;77(3):532–535. doi: 10.1104/pp.77.3.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Varner J. E. An extracellular matrix protein in plants: characterization of a genomic clone for carrot extensin. EMBO J. 1985 Sep;4(9):2145–2151. doi: 10.1002/j.1460-2075.1985.tb03908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland R., Karlsnes A. M. A possible role of hydroxyproline-containing proteins in the cessation of cell elongation. Plant Physiol. 1967 May;42(5):669–671. doi: 10.1104/pp.42.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. B., Schibeci A., Fincher G. B. Biosynthesis of Arabinogalactan-Protein in Lolium multiflorum (Ryegrass) Endosperm Cells : III. Subcellular Distribution of Prolyl Hydroxylase. Plant Physiol. 1983 Jul;72(3):754–758. doi: 10.1104/pp.72.3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker J. R., Davis R. W. Plant defense genes are regulated by ethylene. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5202–5206. doi: 10.1073/pnas.84.15.5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquerré-Tugayé M. T., Lafitte C., Mazau D., Toppan A., Touzé A. Cell Surfaces in Plant-Microorganism Interactions: II. Evidence for the Accumulation of Hydroxyproline-rich Glycoproteins in the Cell Wall of Diseased Plants as a Defense Mechanism. Plant Physiol. 1979 Aug;64(2):320–326. doi: 10.1104/pp.64.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunderson J. H., Elwood H., Ingold A., Kindle K., Sogin M. L. Phylogenetic relationships between chlorophytes, chrysophytes, and oomycetes. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5823–5827. doi: 10.1073/pnas.84.16.5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günzler V., Hanauske-Abel H. M., Myllylä R., Kaska D. D., Hanauske A., Kivirikko K. I. Syncatalytic inactivation of prolyl 4-hydroxylase by anthracyclines. Biochem J. 1988 Apr 15;251(2):365–372. doi: 10.1042/bj2510365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günzler V., Hanauske-Abel H. M., Myllylä R., Mohr J., Kivirikko K. I. Time-dependent inactivation of chick-embryo prolyl 4-hydroxylase by coumalic acid. Evidence for a syncatalytic mechanism. Biochem J. 1987 Feb 15;242(1):163–169. doi: 10.1042/bj2420163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaska D. D., Günzler V., Kivirikko K. I., Myllylä R. Characterization of a low-relative-molecular-mass prolyl 4-hydroxylase from the green alga Chlamydomonas reinhardii. Biochem J. 1987 Jan 15;241(2):483–490. doi: 10.1042/bj2410483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk D. L., Harper J. F. Genetic, biochemical, and molecular approaches to Volvox development and evolution. Int Rev Cytol. 1986;99:217–293. doi: 10.1016/s0074-7696(08)61428-x. [DOI] [PubMed] [Google Scholar]
- Kirk D. L., Kirk M. M. Protein synthetic patterns during the asexual life cycle of Volvox carteri. Dev Biol. 1983 Apr;96(2):493–506. doi: 10.1016/0012-1606(83)90186-0. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Myllylä R. Post-translational processing of procollagens. Ann N Y Acad Sci. 1985;460:187–201. doi: 10.1111/j.1749-6632.1985.tb51167.x. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Myllylä R. Recent developments in posttranslational modification: intracellular processing. Methods Enzymol. 1987;144:96–114. doi: 10.1016/0076-6879(87)44175-x. [DOI] [PubMed] [Google Scholar]
- Klis F. M., Samson M. R., Touw E., Musgrave A., van den Ende H. Sexual Agglutination in the Unicellular Green Alga Chlamydomonas eugametos: Identification and Properties of the Mating Type plus Agglutination Factor. Plant Physiol. 1985 Nov;79(3):740–745. doi: 10.1104/pp.79.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivu J., Myllylä R., Helaakoski T., Pihlajaniemi T., Tasanen K., Kivirikko K. I. A single polypeptide acts both as the beta subunit of prolyl 4-hydroxylase and as a protein disulfide-isomerase. J Biol Chem. 1987 May 15;262(14):6447–6449. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Majamaa K., Günzler V., Hanauske-Abel H. M., Myllylä R., Kivirikko K. I. Partial identity of the 2-oxoglutarate and ascorbate binding sites of prolyl 4-hydroxylase. J Biol Chem. 1986 Jun 15;261(17):7819–7823. [PubMed] [Google Scholar]
- Majamaa K., Hanauske-Abel H. M., Günzler V., Kivirikko K. I. The 2-oxoglutarate binding site of prolyl 4-hydroxylase. Identification of distinct subsites and evidence for 2-oxoglutarate decarboxylation in a ligand reaction at the enzyme-bound ferrous ion. Eur J Biochem. 1984 Jan 16;138(2):239–245. doi: 10.1111/j.1432-1033.1984.tb07907.x. [DOI] [PubMed] [Google Scholar]
- Pihlajaniemi T., Helaakoski T., Tasanen K., Myllylä R., Huhtala M. L., Koivu J., Kivirikko K. I. Molecular cloning of the beta-subunit of human prolyl 4-hydroxylase. This subunit and protein disulphide isomerase are products of the same gene. EMBO J. 1987 Mar;6(3):643–649. doi: 10.1002/j.1460-2075.1987.tb04803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadava D., Chrispeels M. J. Hydroxyproline biosynthesis in plant cells. Peptidyl proline hydroxylase from carrot disks. Biochim Biophys Acta. 1971 Feb 10;227(2):278–287. doi: 10.1016/0005-2744(71)90060-x. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Sato K., Uchida T. Plant prolyl hydroxylase recognizes poly(L-proline) II helix. J Biol Chem. 1981 Nov 25;256(22):11397–11400. [PubMed] [Google Scholar]
- Tanaka M., Shibata H., Uchida T. A new prolyl hydroxylase acting on poly-L-proline, from suspension cultured cells of Vinca rosea. Biochim Biophys Acta. 1980 Dec 4;616(2):188–198. doi: 10.1016/0005-2744(80)90137-0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- de Waal A., Hartog A. F., de Jong L. Photoaffinity labelling of the 2-oxoglutarate binding site of prolyl 4-hydroxylase with 5-azidopyridine-2-carboxylic acid. Biochim Biophys Acta. 1987 Mar 18;912(1):151–155. doi: 10.1016/0167-4838(87)90260-3. [DOI] [PubMed] [Google Scholar]
- de Waal A., de Jong L., Hartog A. F., Kemp A. Photoaffinity labeling of peptide binding sites of prolyl 4-hydroxylase with N-(4-azido-2-nitrophenyl)glycyl-(Pro-Pro-Gly)5. Biochemistry. 1985 Nov 5;24(23):6493–6499. doi: 10.1021/bi00344a028. [DOI] [PubMed] [Google Scholar]