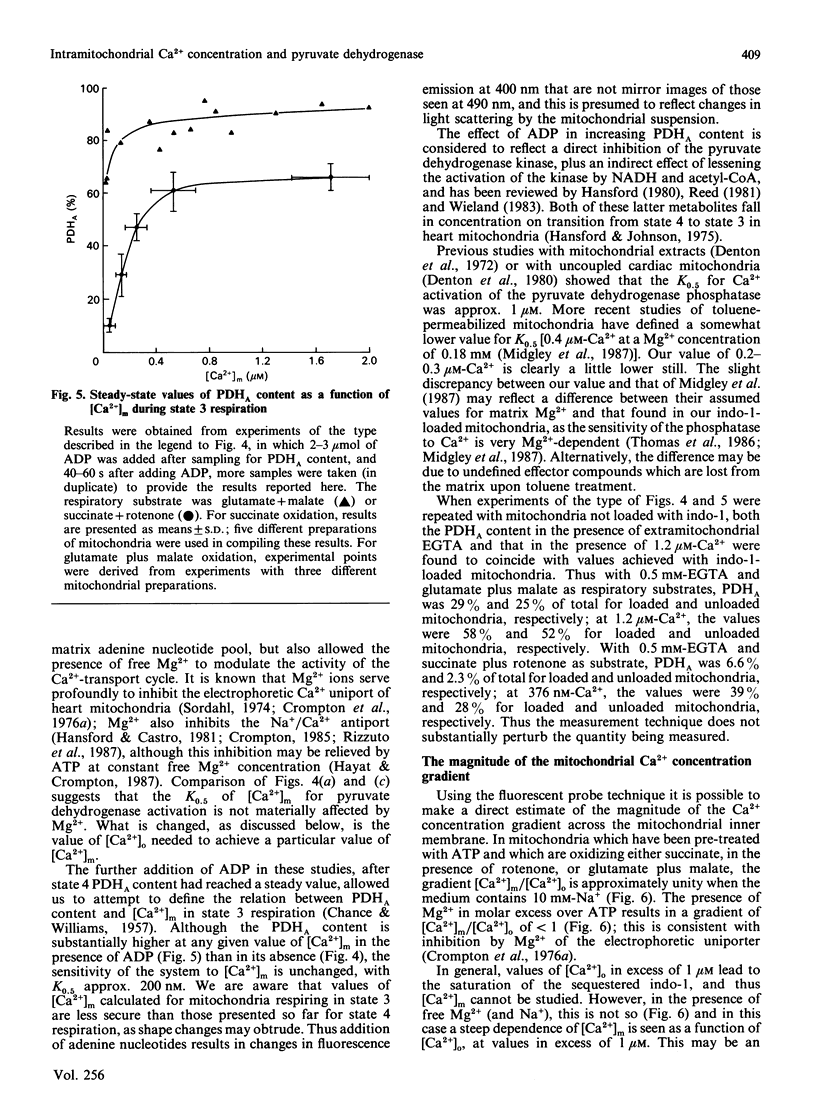
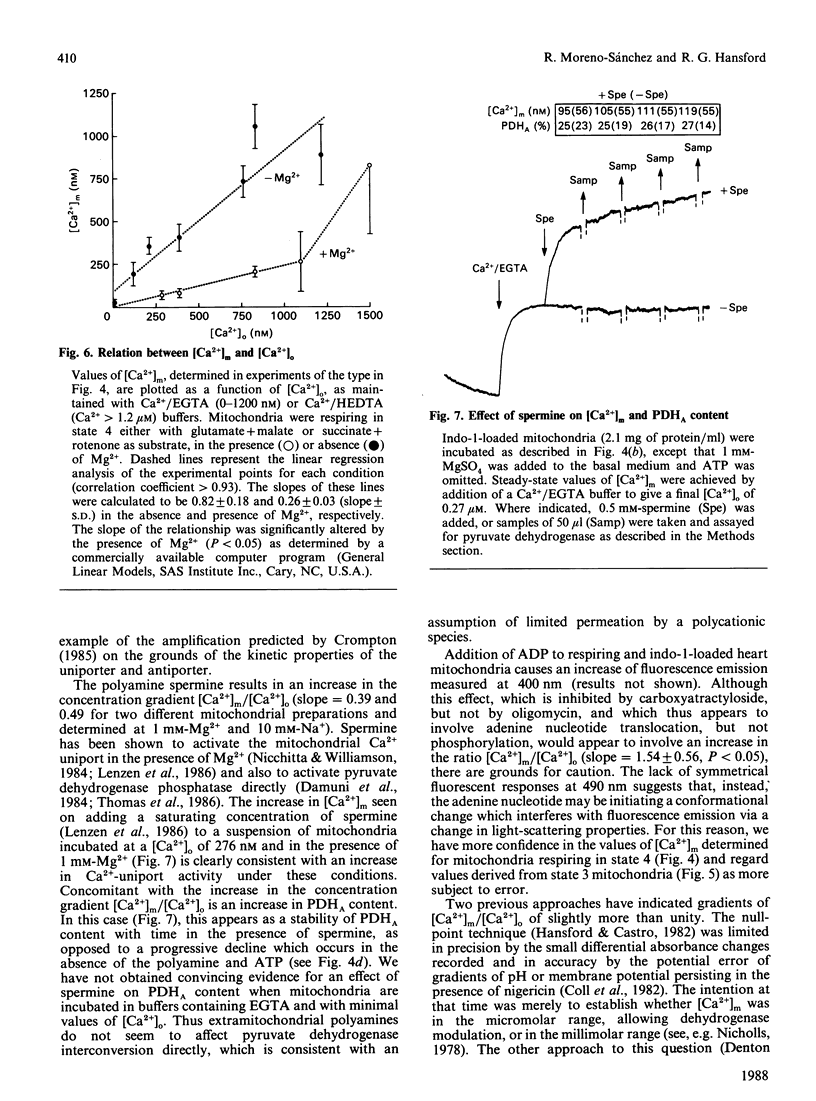
Abstract
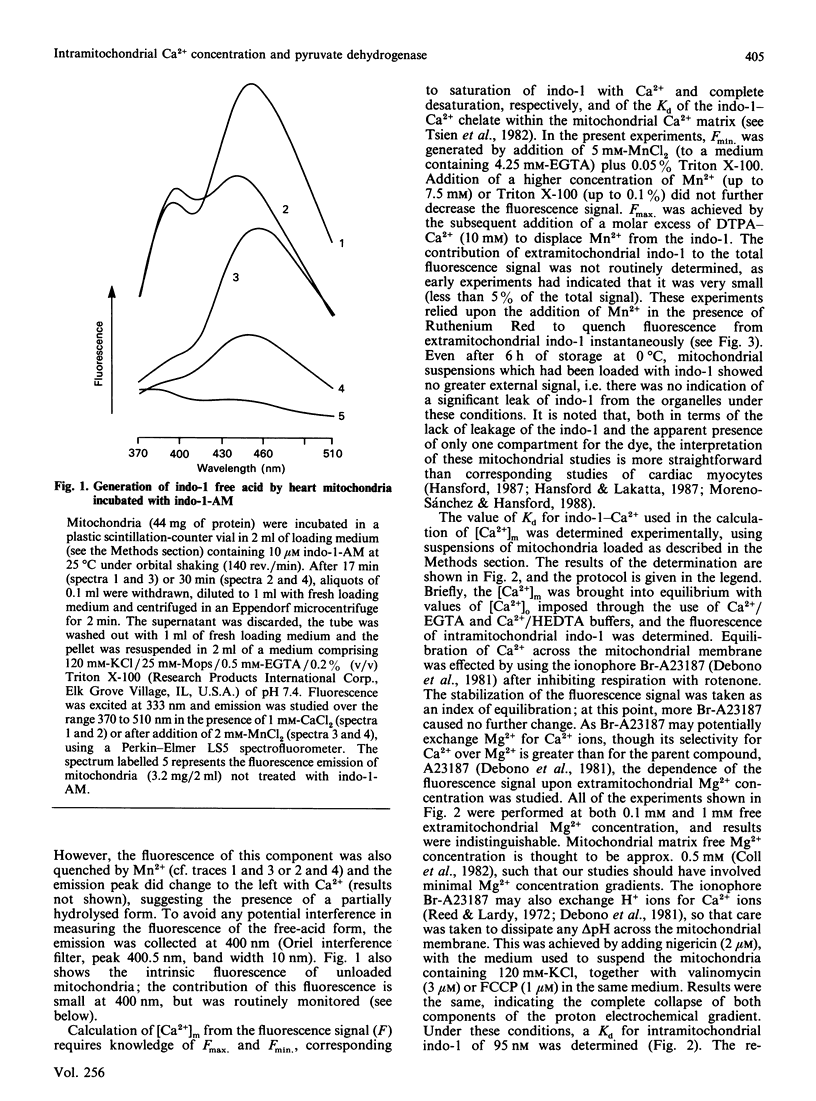
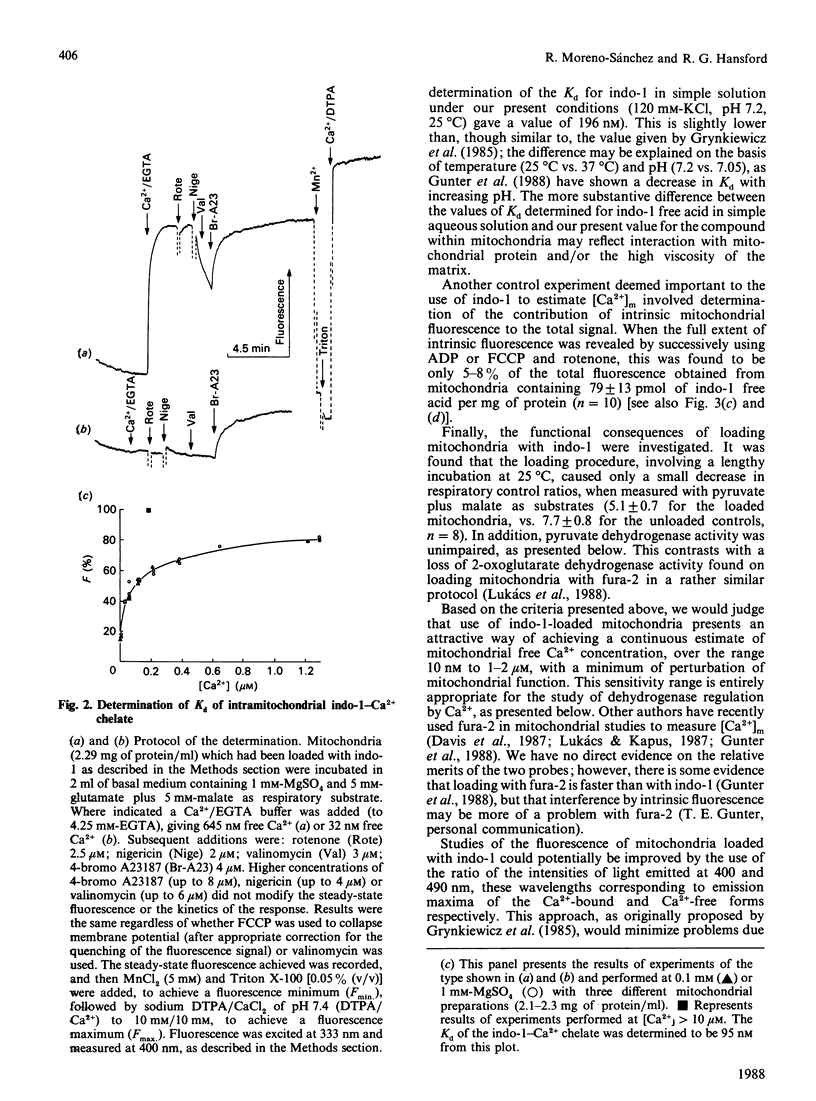
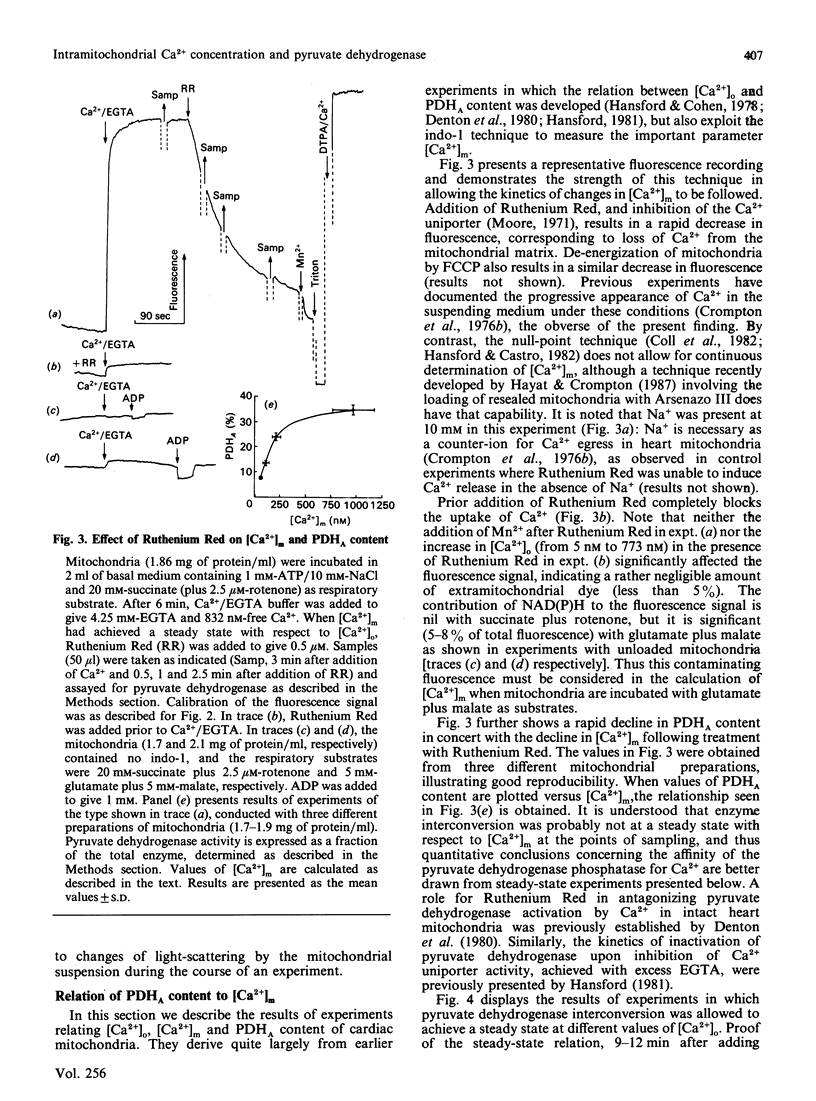
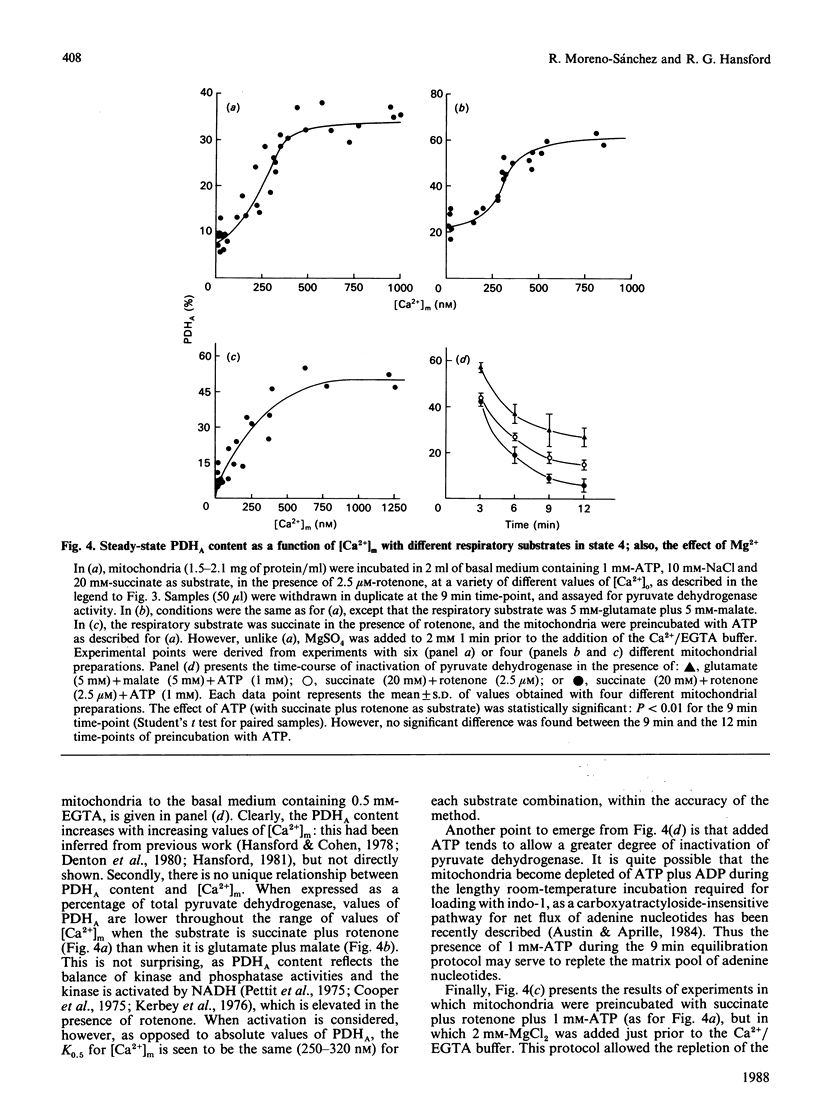
(1) The free Ca2+ concentration of the matrix of rat heart mitochondria ([Ca2+]m) was determined from the fluorescence of internalized indo-1. The value of the Kd of indo-1-Ca2+ in the mitochondrial matrix was determined to be 95 nM, on the basis of equilibration of [Ca2+]m with the extramitochondrial free Ca2+ ([Ca2+]o) in the presence of rotenone, nigericin, valinomycin and Br-A23187. (2) [Ca2+]m responded to energization/de-energization protocols, the inhibition of Ca2+-uptake by Ruthenium Red and the potentiation of Ca2+-efflux by Na+ in a manner which was consistent with the known kinetic properties of the mitochondrial Ca2+-transport processes. (3) The concentration gradient [Ca2+]m/[Ca2+]o was found to be near unity (0.82 +/- 0.18) when mitochondria were incubated in media containing 10 mM-Na+; the additional presence of 1 mM-Mg2+ reduced the gradient to values below unity (0.26 +/- 0.03). The polyamine spermine increased the Ca2+ concentration gradient in the presence of 1 mM-Mg2+. (4) The fraction of pyruvate dehydrogenase in the active form (PDHA) was found to increase with [Ca2+]m, with a K0.5 for activation of approximately 300 nM-Ca2+. This value of the activation constant was not affected by conditions, e.g. addition of Mg2+, which changed the [Ca2+]m/[Ca2+]o concentration gradient, and the presence of different oxidizable substrates, which changed the [NADH/NAD+]m concentration ratio. Thus pyruvate dehydrogenase interconversion responds directly to changes in [Ca2+]m, as inferred in earlier work.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin J., Aprille J. R. Carboxyatractyloside-insensitive influx and efflux of adenine nucleotides in rat liver mitochondria. J Biol Chem. 1984 Jan 10;259(1):154–160. [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- Coll K. E., Joseph S. K., Corkey B. E., Williamson J. R. Determination of the matrix free Ca2+ concentration and kinetics of Ca2+ efflux in liver and heart mitochondria. J Biol Chem. 1982 Aug 10;257(15):8696–8704. [PubMed] [Google Scholar]
- Cooper R. H., Randle P. J., Denton R. M. Stimulation of phosphorylation and inactivation of pyruvate dehydrogenase by physiological inhibitors of the pyruvate dehydrogenase reaction. Nature. 1975 Oct 30;257(5529):808–809. doi: 10.1038/257808a0. [DOI] [PubMed] [Google Scholar]
- Damuni Z., Humphreys J. S., Reed L. J. Stimulation of pyruvate dehydrogenase phosphatase activity by polyamines. Biochem Biophys Res Commun. 1984 Oct 15;124(1):95–99. doi: 10.1016/0006-291x(84)90921-5. [DOI] [PubMed] [Google Scholar]
- Davis M. H., Altschuld R. A., Jung D. W., Brierley G. P. Estimation of intramitochondrial pCa and pH by fura-2 and 2,7 biscarboxyethyl-5(6)-carboxyfluorescein (BCECF) fluorescence. Biochem Biophys Res Commun. 1987 Nov 30;149(1):40–45. doi: 10.1016/0006-291x(87)91602-0. [DOI] [PubMed] [Google Scholar]
- Debono M., Molloy R. M., Dorman D. E., Paschal J. W., Babcock D. F., Deber C. M., Pfeiffer D. R. Synthesis and characterization of halogenated derivatives of the ionophore A23187: enhanced calcium ion transport specificity by the 4-bromo derivative. Biochemistry. 1981 Nov 24;20(24):6865–6872. doi: 10.1021/bi00527a019. [DOI] [PubMed] [Google Scholar]
- Denton R. M., McCormack J. G. Ca2+ transport by mammalian mitochondria and its role in hormone action. Am J Physiol. 1985 Dec;249(6 Pt 1):E543–E554. doi: 10.1152/ajpendo.1985.249.6.E543. [DOI] [PubMed] [Google Scholar]
- Denton R. M., McCormack J. G., Edgell N. J. Role of calcium ions in the regulation of intramitochondrial metabolism. Effects of Na+, Mg2+ and ruthenium red on the Ca2+-stimulated oxidation of oxoglutarate and on pyruvate dehydrogenase activity in intact rat heart mitochondria. Biochem J. 1980 Jul 15;190(1):107–117. doi: 10.1042/bj1900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton R. M., McCormack J. G. On the role of the calcium transport cycle in heart and other mammalian mitochondria. FEBS Lett. 1980 Sep 22;119(1):1–8. doi: 10.1016/0014-5793(80)80986-0. [DOI] [PubMed] [Google Scholar]
- Denton R. M., Randle P. J., Martin B. R. Stimulation by calcium ions of pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1972 Jun;128(1):161–163. doi: 10.1042/bj1280161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton R. M., Richards D. A., Chin J. G. Calcium ions and the regulation of NAD+-linked isocitrate dehydrogenase from the mitochondria of rat heart and other tissues. Biochem J. 1978 Dec 15;176(3):899–906. doi: 10.1042/bj1760899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hansford R. G., Castro F. Effects of micromolar concentrations of free calcium ions on the reduction of heart mitochondrial NAD(P) by 2-oxoglutarate. Biochem J. 1981 Sep 15;198(3):525–533. doi: 10.1042/bj1980525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansford R. G., Castro F. Intramitochondrial and extramitochondrial free calcium ion concentrations of suspensions of heart mitochondria with very low, plausibly physiological, contents of total calcium. J Bioenerg Biomembr. 1982 Dec;14(5-6):361–376. doi: 10.1007/BF00743064. [DOI] [PubMed] [Google Scholar]
- Hansford R. G., Cohen L. Relative importance of pyruvate dehydrogenase interconversion and feed-back inhibition in the effect of fatty acids on pyruvate oxidation by rat heart mitochondria. Arch Biochem Biophys. 1978 Nov;191(1):65–81. doi: 10.1016/0003-9861(78)90068-1. [DOI] [PubMed] [Google Scholar]
- Hansford R. G. Effect of micromolar concentrations of free Ca2+ ions on pyruvate dehydrogenase interconversion in intact rat heart mitochondria. Biochem J. 1981 Mar 15;194(3):721–732. doi: 10.1042/bj1940721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansford R. G., Johnson R. N. The steady state concentrations of coenzyme A-SH and coenzyme A thioester, citrate, and isocitrate during tricarboxylate cycle oxidations in rabbit heart mitochondria. J Biol Chem. 1975 Nov 10;250(21):8361–8375. [PubMed] [Google Scholar]
- Hansford R. G., Lakatta E. G. Ryanodine releases calcium from sarcoplasmic reticulum in calcium-tolerant rat cardiac myocytes. J Physiol. 1987 Sep;390:453–467. doi: 10.1113/jphysiol.1987.sp016711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansford R. G. Lipid oxidation by heart mitochondria from young adult and senescent rats. Biochem J. 1978 Feb 15;170(2):285–295. doi: 10.1042/bj1700285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansford R. G. Relation between cytosolic free Ca2+ concentration and the control of pyruvate dehydrogenase in isolated cardiac myocytes. Biochem J. 1987 Jan 1;241(1):145–151. doi: 10.1042/bj2410145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansford R. G. Relation between mitochondrial calcium transport and control of energy metabolism. Rev Physiol Biochem Pharmacol. 1985;102:1–72. doi: 10.1007/BFb0034084. [DOI] [PubMed] [Google Scholar]
- Hansford R. G. Studies on inactivation of pyruvate dehydrogenase by palmitoylcarnitine oxidation in isolated rat heart mitochondria. J Biol Chem. 1977 Mar 10;252(5):1552–1560. [PubMed] [Google Scholar]
- Hansford R. G. Studies on the effects of coenzyme A-SH: acetyl coenzyme A, nicotinamide adenine dinucleotide: reduced nicotinamide adenine dinucleotide, and adenosine diphosphate: adenosine triphosphate ratios on the interconversion of active and inactive pyruvate dehydrogenase in isolated rat heart mitochondria. J Biol Chem. 1976 Sep 25;251(18):5483–5489. [PubMed] [Google Scholar]
- Hayat L. H., Crompton M. The effects of Mg2+ and adenine nucleotides on the sensitivity of the heart mitochondrial Na+-Ca2+ carrier to extramitochondrial Ca2+. A study using arsenazo III-loaded mitochondria. Biochem J. 1987 Jun 15;244(3):533–538. doi: 10.1042/bj2440533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz A. M. Contractile proteins of the heart. Physiol Rev. 1970 Jan;50(1):63–158. doi: 10.1152/physrev.1970.50.1.63. [DOI] [PubMed] [Google Scholar]
- Kerbey A. L., Randle P. J., Cooper R. H., Whitehouse S., Pask H. T., Denton R. M. Regulation of pyruvate dehydrogenase in rat heart. Mechanism of regulation of proportions of dephosphorylated and phosphorylated enzyme by oxidation of fatty acids and ketone bodies and of effects of diabetes: role of coenzyme A, acetyl-coenzyme A and reduced and oxidized nicotinamide-adenine dinucleotide. Biochem J. 1976 Feb 15;154(2):327–348. doi: 10.1042/bj1540327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koretsky A. P., Balaban R. S. Changes in pyridine nucleotide levels alter oxygen consumption and extra-mitochondrial phosphates in isolated mitochondria: a 31P-NMR and NAD(P)H fluorescence study. Biochim Biophys Acta. 1987 Oct 7;893(3):398–408. doi: 10.1016/0005-2728(87)90092-2. [DOI] [PubMed] [Google Scholar]
- Lawlis V. B., Roche T. E. Effect of micromolar Ca2+ on NADH inhibition of bovine kidney alpha-ketoglutarate dehydrogenase complex and possible role of Ca2+ in signal amplification. Mol Cell Biochem. 1980 Nov 20;32(3):147–152. doi: 10.1007/BF00227441. [DOI] [PubMed] [Google Scholar]
- Lehninger A. L., Carafoli E., Rossi C. S. Energy-linked ion movements in mitochondrial systems. Adv Enzymol Relat Areas Mol Biol. 1967;29:259–320. doi: 10.1002/9780470122747.ch6. [DOI] [PubMed] [Google Scholar]
- Lenzen S., Hickethier R., Panten U. Interactions between spermine and Mg2+ on mitochondrial Ca2+ transport. J Biol Chem. 1986 Dec 15;261(35):16478–16483. [PubMed] [Google Scholar]
- Lukács G. L., Kapus A., Fonyó A. Parallel measurement of oxoglutarate dehydrogenase activity and matrix free Ca2+ in fura-2-loaded heart mitochondria. FEBS Lett. 1988 Feb 29;229(1):219–223. doi: 10.1016/0014-5793(88)80831-7. [DOI] [PubMed] [Google Scholar]
- Lukács G. L., Kapus A. Measurement of the matrix free Ca2+ concentration in heart mitochondria by entrapped fura-2 and quin2. Biochem J. 1987 Dec 1;248(2):609–613. doi: 10.1042/bj2480609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack J. G., Denton R. M. The effects of calcium ions and adenine nucleotides on the activity of pig heart 2-oxoglutarate dehydrogenase complex. Biochem J. 1979 Jun 15;180(3):533–544. doi: 10.1042/bj1800533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack J. G., England P. J. Ruthenium Red inhibits the activation of pyruvate dehydrogenase caused by positive inotropic agents in the perfused rat heart. Biochem J. 1983 Aug 15;214(2):581–585. doi: 10.1042/bj2140581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midgley P. J., Rutter G. A., Thomas A. P., Denton R. M. Effects of Ca2+ and Mg2+ on the activity of pyruvate dehydrogenase phosphate phosphatase within toluene-permeabilized mitochondria. Biochem J. 1987 Jan 15;241(2):371–377. doi: 10.1042/bj2410371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C. L. Specific inhibition of mitochondrial Ca++ transport by ruthenium red. Biochem Biophys Res Commun. 1971 Jan 22;42(2):298–305. doi: 10.1016/0006-291x(71)90102-1. [DOI] [PubMed] [Google Scholar]
- Moreno-Sánchez R., Hansford R. G. Relation between cytosolic free calcium and respiratory rates in cardiac myocytes. Am J Physiol. 1988 Aug;255(2 Pt 2):H347–H357. doi: 10.1152/ajpheart.1988.255.2.H347. [DOI] [PubMed] [Google Scholar]
- Nicchitta C. V., Williamson J. R. Spermine. A regulator of mitochondrial calcium cycling. J Biol Chem. 1984 Nov 10;259(21):12978–12983. [PubMed] [Google Scholar]
- Nicholls D. G. The influence of respiration and ATP hydrolysis on the proton-electrochemical gradient across the inner membrane of rat-liver mitochondria as determined by ion distribution. Eur J Biochem. 1974 Dec 16;50(1):305–315. doi: 10.1111/j.1432-1033.1974.tb03899.x. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G. The regulation of extramitochondrial free calcium ion concentration by rat liver mitochondria. Biochem J. 1978 Nov 15;176(2):463–474. doi: 10.1042/bj1760463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit F. H., Pelley J. W., Reed L. J. Regulation of pyruvate dehydrogenase kinase and phosphatase by acetyl-CoA/CoA and NADH/NAD ratios. Biochem Biophys Res Commun. 1975 Jul 22;65(2):575–582. doi: 10.1016/s0006-291x(75)80185-9. [DOI] [PubMed] [Google Scholar]
- Pettit F. H., Roche T. E., Reed L. J. Function of calcium ions in pyruvate dehydrogenase phosphatase activity. Biochem Biophys Res Commun. 1972 Oct 17;49(2):563–571. doi: 10.1016/0006-291x(72)90448-2. [DOI] [PubMed] [Google Scholar]
- Reed L. J. Regulation of mammalian pyruvate dehydrogenase complex by a phosphorylation-dephosphorylation cycle. Curr Top Cell Regul. 1981;18:95–106. doi: 10.1016/b978-0-12-152818-8.50012-8. [DOI] [PubMed] [Google Scholar]
- Reed P. W., Lardy H. A. A23187: a divalent cation ionophore. J Biol Chem. 1972 Nov 10;247(21):6970–6977. [PubMed] [Google Scholar]
- Rizzuto R., Bernardi P., Favaron M., Azzone G. F. Pathways for Ca2+ efflux in heart and liver mitochondria. Biochem J. 1987 Sep 1;246(2):271–277. doi: 10.1042/bj2460271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu S. S., Fozzard H. A. Transmembrane Na+ and Ca2+ electrochemical gradients in cardiac muscle and their relationship to force development. J Gen Physiol. 1982 Sep;80(3):325–351. doi: 10.1085/jgp.80.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordahl L. A. Effects of magnesium, Ruthenium red and the antibiotic ionophore A-23187 on initial rates of calcium uptake and release by heart mitochondria. Arch Biochem Biophys. 1975 Mar;167(1):104–115. doi: 10.1016/0003-9861(75)90446-4. [DOI] [PubMed] [Google Scholar]
- Thomas A. P., Diggle T. A., Denton R. M. Sensitivity of pyruvate dehydrogenase phosphate phosphatase to magnesium ions. Similar effects of spermine and insulin. Biochem J. 1986 Aug 15;238(1):83–91. doi: 10.1042/bj2380083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland O. H. The mammalian pyruvate dehydrogenase complex: structure and regulation. Rev Physiol Biochem Pharmacol. 1983;96:123–170. doi: 10.1007/BFb0031008. [DOI] [PubMed] [Google Scholar]


