Abstract
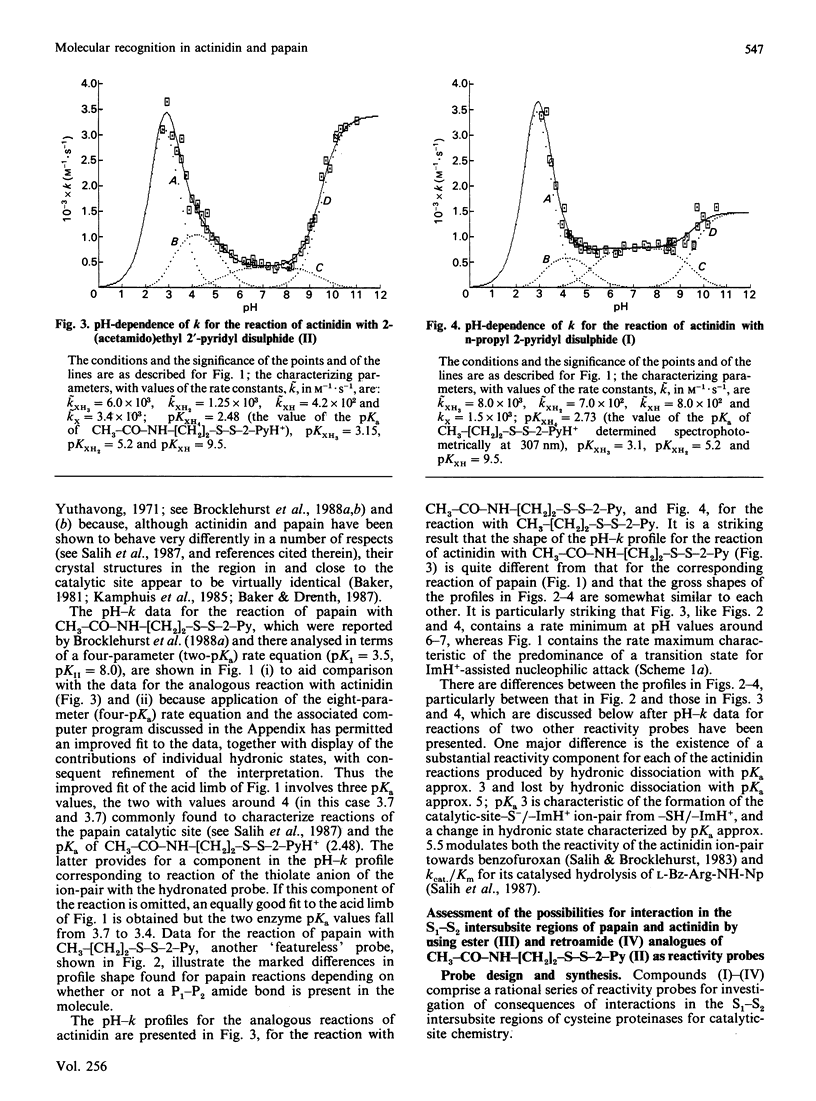
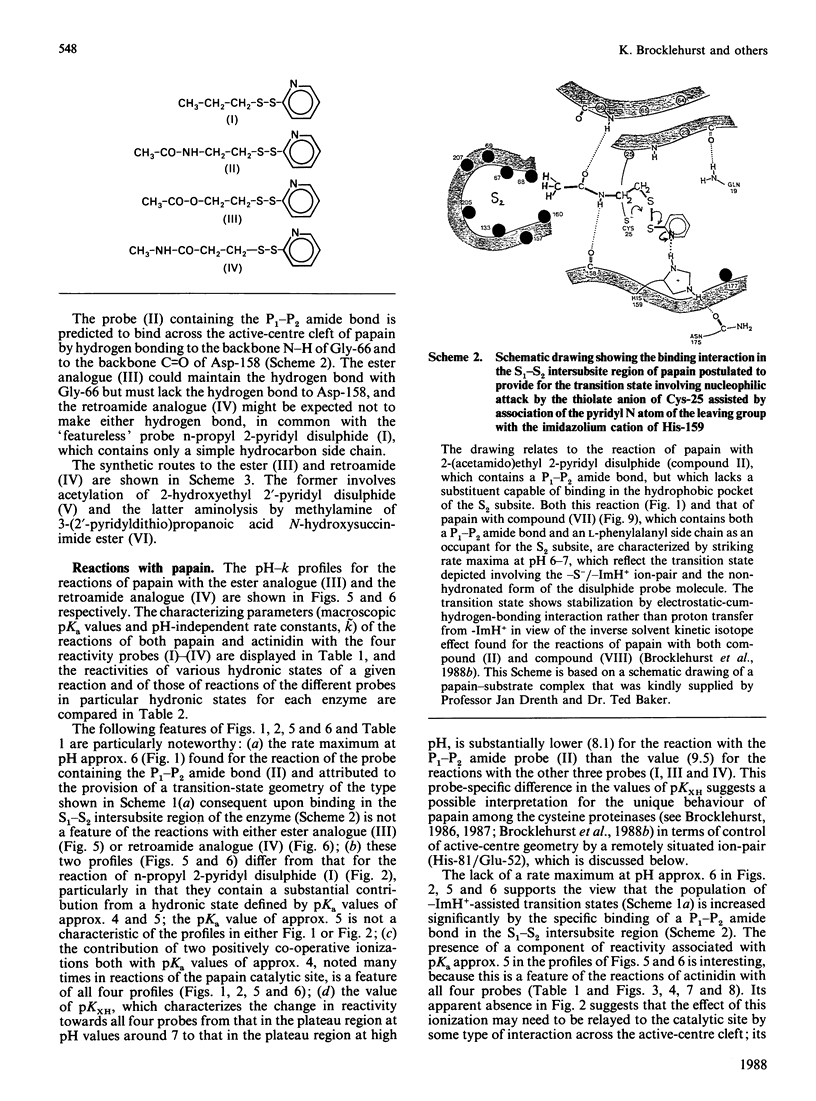
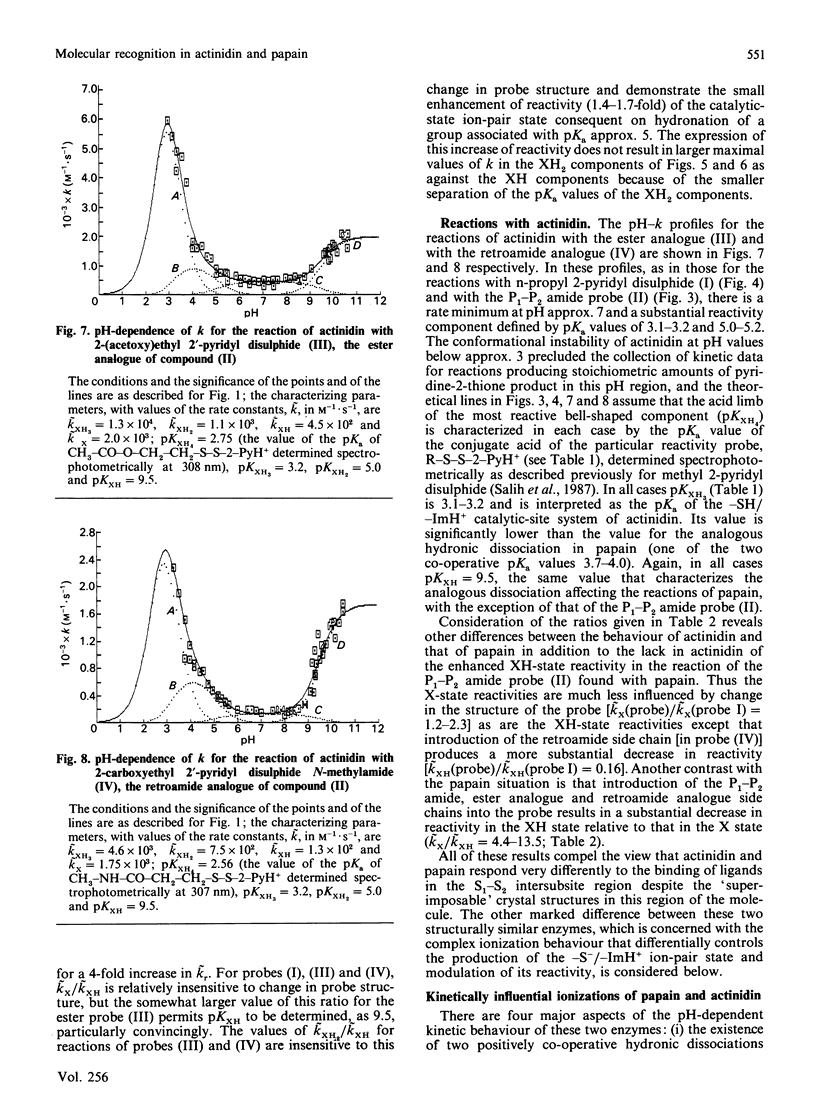
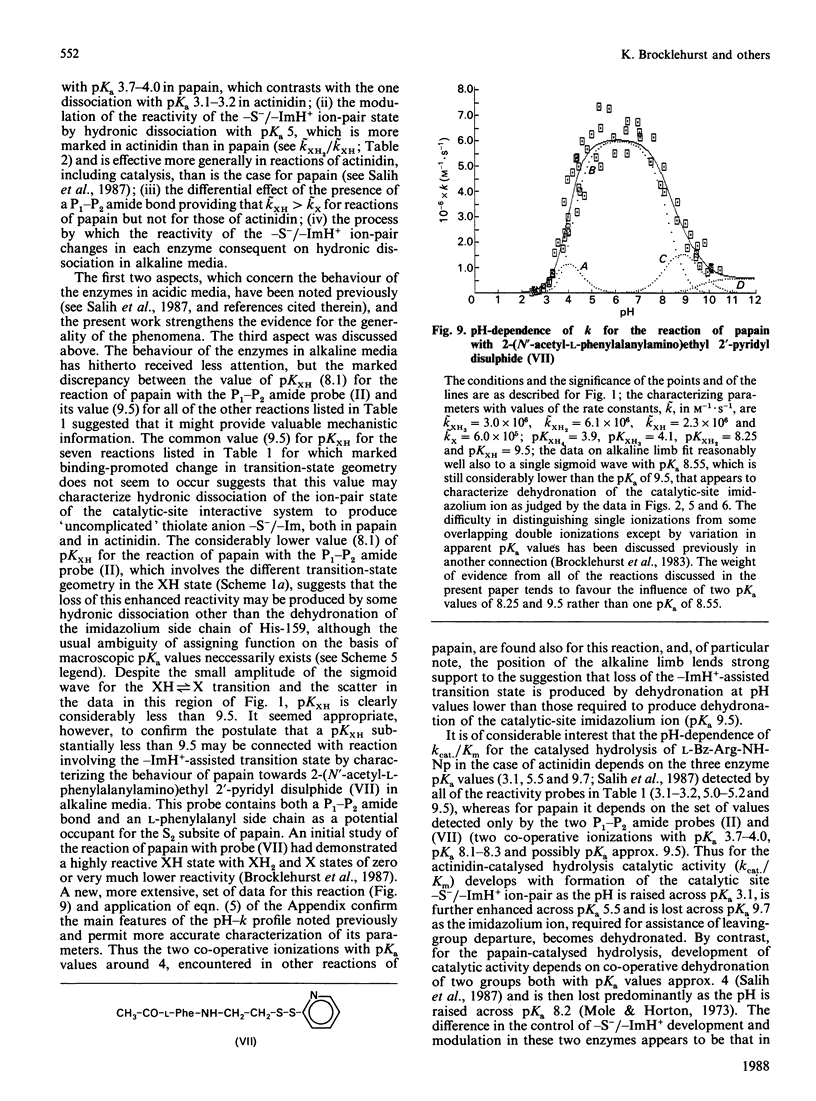
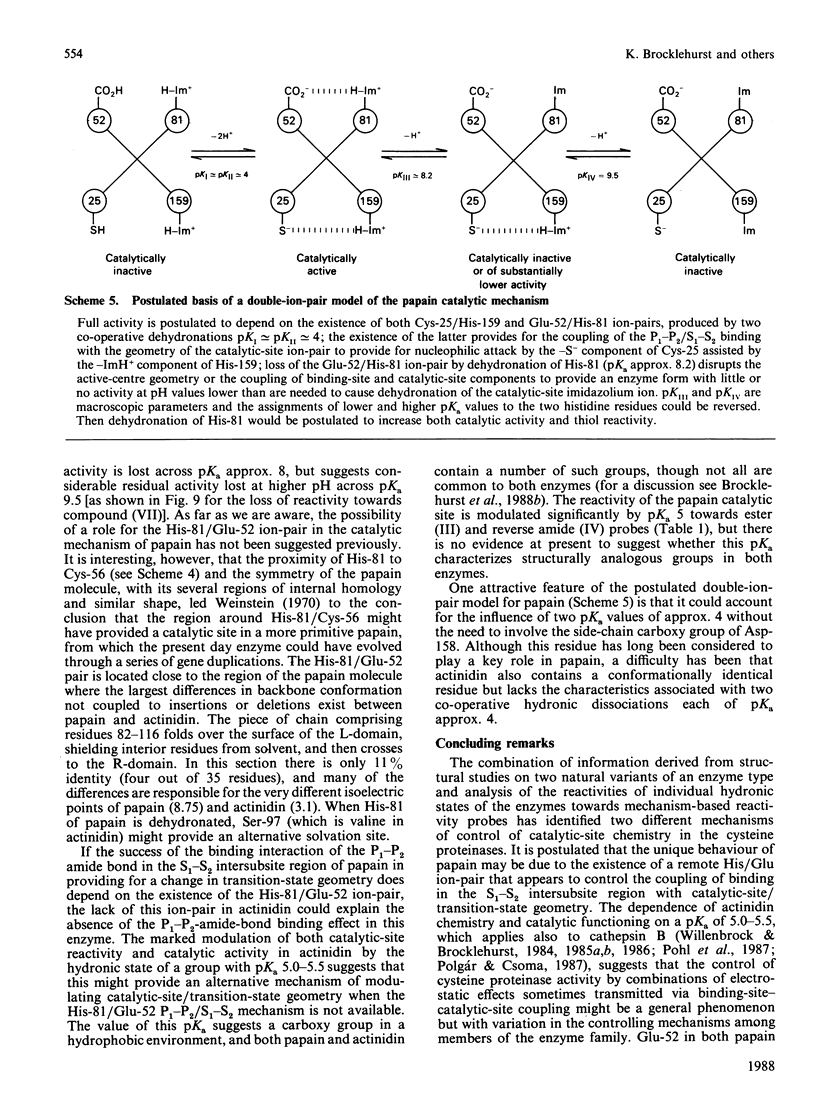
1. The influence on the reactivities of the catalytic sites of papain (EC 3.4.22.2) and actinidin (3.4.22.14) of providing for interactions involving the S1-S2 intersubsite regions of the enzymes was evaluated by using as a series of thiol-specific two-hydronic-state reactivity probes: n-propyl 2-pyridyl disulphide (I) (a 'featureless' probe), 2-(acetamido)ethyl 2'-pyridyl disulphide (II) (containing a P1-P2 amide bond), 2-(acetoxy)ethyl 2'-pyridyl disulphide (III) [the ester analogue of probe (II)] and 2-carboxyethyl 2'-pyridyl disulphide N-methylamide (IV) [the retroamide analogue of probe (II)]. Syntheses of compounds (I), (III) and (IV) are reported. 2. The reactivities of the two enzymes towards the four reactivity probes (I)-(IV) and also that of papain towards 2-(N'-acetyl-L-phenylalanylamino)ethyl 2'-pyridyl disulphide (VII) (containing both a P1-P2 amide bond and an L-phenylalanyl side chain as an occupant for the S2 subsite), in up to four hydronic (previously called protonic) states, were evaluated by analysis of pH-dependent stopped-flow kinetic data (for the release of pyridine-2-thione) by using an eight-parameter rate equation [described in the Appendix: Brocklehurst & Brocklehurst (1988) Biochem. J. 256, 556-558] to provide pH-independent rate constants and macroscopic pKa values. The analysis reveals the various ways in which the two enzymes respond very differently to the binding of ligands in the S1-S2 intersubsite regions despite the virtually superimposable crystal structures in these regions of the molecules. 3. Particularly striking differences between the behaviour of papain and that of actinidin are that (a) only papain responds to the presence of a P1-P2 amide bond in the probe such that a rate maximum at pH 6-7 is produced in the pH-k profile in place of the rate minimum, (b) only in the papain reactions does the pKa value of the alkaline limb of the pH-k profile change from 9.5 to approx. 8.2 [the value characteristic of a pH-(kcat./Km) profile] when the probe contains a P1-P2 amide bond, (c) only papain reactivity is affected by two positively co-operative hydronic dissociations with pKI congruent to pKII congruent to 4 and (d) modulation of the reactivity of the common -S(-)-ImH+ catalytic-site ion-pair (Cys-25/His-159 in papain and Cys-25/His-162 in actinidin) by hydronic dissociation with pKa approx. 5 is more marked and occurs more generally in reactions of actinidin than is the case for papain reactions.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baines B. S., Brocklehurst K. A necessary modification to the preparation of papain from any high-quality latex of Carica papaya and evidence for the structural integrity of the enzyme produced by traditional methods. Biochem J. 1979 Feb 1;177(2):541–548. doi: 10.1042/bj1770541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger A., Schechter I. Mapping the active site of papain with the aid of peptide substrates and inhibitors. Philos Trans R Soc Lond B Biol Sci. 1970 Feb 12;257(813):249–264. doi: 10.1098/rstb.1970.0024. [DOI] [PubMed] [Google Scholar]
- Blundell T., Carney D., Gardner S., Hayes F., Howlin B., Hubbard T., Overington J., Singh D. A., Sibanda B. L., Sutcliffe M. 18th Sir Hans Krebs lecture. Knowledge-based protein modelling and design. Eur J Biochem. 1988 Mar 15;172(3):513–520. doi: 10.1111/j.1432-1033.1988.tb13917.x. [DOI] [PubMed] [Google Scholar]
- Brocklehurst K., Baines B. S., Malthouse J. P. Differences in the interaction of the catalytic groups of the active centres of actinidin and papain. Rapid purification of fully active actinidin by covalent chromatography and characterization of its active centre by use of two-protonic-state reactivity probes. Biochem J. 1981 Sep 1;197(3):739–746. doi: 10.1042/bj1970739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Kowlessur D., O'Driscoll M., Patel G., Quenby S., Salih E., Templeton W., Thomas E. W., Willenbrock F. Substrate-derived two-protonic-state electrophiles as sensitive kinetic specificity probes for cysteine proteinases. Activation of 2-pyridyl disulphides by hydrogen-bonding. Biochem J. 1987 May 15;244(1):173–181. doi: 10.1042/bj2440173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Kowlessur D., Patel G., Templeton W., Quigley K., Thomas E. W., Wharton C. W., Willenbrock F., Szawelski R. J. Consequences of molecular recognition in the S1-S2 intersubsite region of papain for catalytic-site chemistry. Change in pH-dependence characteristics and generation of an inverse solvent kinetic isotope effect by introduction of a P1-P2 amide bond into a two-protonic-state reactivity probe. Biochem J. 1988 Mar 15;250(3):761–772. doi: 10.1042/bj2500761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Little G. Reactions of papain and of low-molecular-weight thiols with some aromatic disulphides. 2,2'-Dipyridyl disulphide as a convenient active-site titrant for papain even in the presence of other thiols. Biochem J. 1973 May;133(1):67–80. doi: 10.1042/bj1330067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Willenbrock S. J., Salih E. Effects of conformational selectivity and of overlapping kinetically influential ionizations on the characteristics of pH-dependent enzyme kinetics. Implications of free-enzyme pKa variability in reactions of papain for its catalytic mechanism. Biochem J. 1983 Jun 1;211(3):701–708. doi: 10.1042/bj2110701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst S. M., Brocklehurst K. Appendix: Analysis of pH-dependent kinetics in up to four reactive hydronic states. Biochem J. 1988 Dec 1;256(2):556–558. doi: 10.1042/bj2560556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J., Drevin H., Axén R. Protein thiolation and reversible protein-protein conjugation. N-Succinimidyl 3-(2-pyridyldithio)propionate, a new heterobifunctional reagent. Biochem J. 1978 Sep 1;173(3):723–737. doi: 10.1042/bj1730723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik C. S., Largman C., Fletcher T., Roczniak S., Barr P. J., Fletterick R., Rutter W. J. Redesigning trypsin: alteration of substrate specificity. Science. 1985 Apr 19;228(4697):291–297. doi: 10.1126/science.3838593. [DOI] [PubMed] [Google Scholar]
- Estell D. A., Graycar T. P., Miller J. V., Powers D. B., Wells J. A., Burnier J. P., Ng P. G. Probing steric and hydrophobic effects on enzyme-substrate interactions by protein engineering. Science. 1986 Aug 8;233(4764):659–663. doi: 10.1126/science.233.4764.659. [DOI] [PubMed] [Google Scholar]
- Kamphuis I. G., Drenth J., Baker E. N. Thiol proteases. Comparative studies based on the high-resolution structures of papain and actinidin, and on amino acid sequence information for cathepsins B and H, and stem bromelain. J Mol Biol. 1985 Mar 20;182(2):317–329. doi: 10.1016/0022-2836(85)90348-1. [DOI] [PubMed] [Google Scholar]
- Knowles J. R. Tinkering with enzymes: what are we learning? Science. 1987 Jun 5;236(4806):1252–1258. doi: 10.1126/science.3296192. [DOI] [PubMed] [Google Scholar]
- Leatherbarrow R. J., Fersht A. R. Protein engineering. Protein Eng. 1986 Oct-Nov;1(1):7–16. doi: 10.1093/protein/1.1.7. [DOI] [PubMed] [Google Scholar]
- Lowe G., Yuthavong Y. Kinetic specificity in papain-catalysed hydrolyses. Biochem J. 1971 Aug;124(1):107–115. doi: 10.1042/bj1240107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabbutt B. C., Williams R. J. Two-dimensional 1H-NMR studies of the solution structure of plasminogen kringle 4. Eur J Biochem. 1988 Jan 4;170(3):539–548. doi: 10.1111/j.1432-1033.1988.tb13733.x. [DOI] [PubMed] [Google Scholar]
- Mole J. E., Horton H. R. Kinetics of papain-catalyzed hydrolysis of -N-benzoyl-L-arginine-p-nitroanilide. Biochemistry. 1973 Feb 27;12(5):816–822. doi: 10.1021/bi00729a005. [DOI] [PubMed] [Google Scholar]
- Pohl J., Davinic S., Bláha I., Strop P., Kostka V. Chromophoric and fluorophoric peptide substrates cleaved through the dipeptidyl carboxypeptidase activity of cathepsin B. Anal Biochem. 1987 Aug 15;165(1):96–101. doi: 10.1016/0003-2697(87)90205-3. [DOI] [PubMed] [Google Scholar]
- Russell A. J., Thomas P. G., Fersht A. R. Electrostatic effects on modification of charged groups in the active site cleft of subtilisin by protein engineering. J Mol Biol. 1987 Feb 20;193(4):803–813. doi: 10.1016/0022-2836(87)90360-3. [DOI] [PubMed] [Google Scholar]
- Salih E., Brocklehurst K. Investigation of the catalytic site of actinidin by using benzofuroxan as a reactivity probe with selectivity for the thiolate-imidazolium ion-pair systems of cysteine proteinases. Evidence that the reaction of the ion-pair of actinidin (pKI 3.0, pKII 9.6) is modulated by the state of ionization of a group associated with a molecular pKa of 5.5. Biochem J. 1983 Sep 1;213(3):713–718. doi: 10.1042/bj2130713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salih E., Malthouse J. P., Kowlessur D., Jarvis M., O'Driscoll M., Brocklehurst K. Differences in the chemical and catalytic characteristics of two crystallographically 'identical' enzyme catalytic sites. Characterization of actinidin and papain by a combination of pH-dependent substrate catalysis kinetics and reactivity probe studies targeted on the catalytic-site thiol group and its immediate microenvironment. Biochem J. 1987 Oct 1;247(1):181–193. doi: 10.1042/bj2470181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. V. Protein engineering. The design, synthesis and characterization of factitious proteins. Biochem J. 1987 Aug 15;246(1):1–17. doi: 10.1042/bj2460001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipton M., Brochlehurst K. Characterization of the papain active centre by using two-protonic-state electrophiles as reactivity probes. Evidence for nucleophilic reactivity in the un-interrupted cysteine-25-histidine-159 interactive system. Biochem J. 1978 May 1;171(2):385–401. doi: 10.1042/bj1710385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. J., Maggio E. T., Kenyon G. L. Simple alkanethiol groups for temporary blocking of sulfhydryl groups of enzymes. Biochemistry. 1975 Feb 25;14(4):766–771. doi: 10.1021/bi00675a019. [DOI] [PubMed] [Google Scholar]
- Stuchbury T., Shipton M., Norris R., Malthouse J. P., Brocklehurst K., Herbert J. A., Suschitzky H. A reporter group delivery system with both absolute and selective specificity for thiol groups and an improved fluorescent probe containing the 7-nitrobenzo-2-oxa-1,3-diazole moiety. Biochem J. 1975 Nov;151(2):417–432. doi: 10.1042/bj1510417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe M. J., Haneef I., Carney D., Blundell T. L. Knowledge based modelling of homologous proteins, Part I: Three-dimensional frameworks derived from the simultaneous superposition of multiple structures. Protein Eng. 1987 Oct-Nov;1(5):377–384. doi: 10.1093/protein/1.5.377. [DOI] [PubMed] [Google Scholar]
- Sutcliffe M. J., Hayes F. R., Blundell T. L. Knowledge based modelling of homologous proteins, Part II: Rules for the conformations of substituted sidechains. Protein Eng. 1987 Oct-Nov;1(5):385–392. doi: 10.1093/protein/1.5.385. [DOI] [PubMed] [Google Scholar]
- Wand A. J., Englander S. W. Two-dimensional 1H NMR studies of cytochrome c. Biochemistry. 1985 Sep 24;24(20):5290–5294. doi: 10.1021/bi00341a002. [DOI] [PubMed] [Google Scholar]
- Warshel A., Russell S. T. Calculations of electrostatic interactions in biological systems and in solutions. Q Rev Biophys. 1984 Aug;17(3):283–422. doi: 10.1017/s0033583500005333. [DOI] [PubMed] [Google Scholar]
- Warshel A., Sussman F. Toward computer-aided site-directed mutagenesis of enzymes. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3806–3810. doi: 10.1073/pnas.83.11.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein B. The evolution of papain. Biochem Biophys Res Commun. 1970 Oct 23;41(2):441–443. doi: 10.1016/0006-291x(70)90524-3. [DOI] [PubMed] [Google Scholar]
- Wells J. A., Powers D. B., Bott R. R., Graycar T. P., Estell D. A. Designing substrate specificity by protein engineering of electrostatic interactions. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1219–1223. doi: 10.1073/pnas.84.5.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willenbrock F., Brocklehurst K. A general framework of cysteine-proteinase mechanism deduced from studies on enzymes with structurally different analogous catalytic-site residues Asp-158 and -161 (papain and actinidin), Gly-196 (cathepsin B) and Asn-165 (cathepsin H). Kinetic studies up to pH 8 of the hydrolysis of N-alpha-benzyloxycarbonyl-L-arginyl-L-arginine 2-naphthylamide catalysed by cathepsin B and of L-arginine 2-naphthylamide catalysed by cathepsin H. Biochem J. 1985 Apr 15;227(2):521–528. doi: 10.1042/bj2270521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willenbrock F., Brocklehurst K. Chemical evidence for the pH-dependent control of ion-pair geometry in cathepsin B. Benzofuroxan as a reactivity probe sensitive to differences in the mutual disposition of the thiolate and imidazolium components of cysteine proteinase catalytic sites. Biochem J. 1986 Aug 15;238(1):103–107. doi: 10.1042/bj2380103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willenbrock F., Brocklehurst K. Natural structural variation in enzymes as a tool in the study of mechanism exemplified by a comparison of the catalytic-site structure and characteristics of cathepsin B and papain. pH-dependent kinetics of the reactions of cathepsin B from bovine spleen and from rat liver with a thiol-specific two-protonic-state probe (2,2'-dipyridyl disulphide) and with a specific synthetic substrate (N-alpha-benzyloxycarbonyl-L-arginyl-L-arginine 2-naphthylamide). Biochem J. 1984 Sep 15;222(3):805–814. doi: 10.1042/bj2220805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willenbrock F., Brocklehurst K. Preparation of cathepsins B and H by covalent chromatography and characterization of their catalytic sites by reaction with a thiol-specific two-protonic-state reactivity probe. Kinetic study of cathepsins B and H extending into alkaline media and a rapid spectroscopic titration of cathepsin H at pH 3-4. Biochem J. 1985 Apr 15;227(2):511–519. doi: 10.1042/bj2270511. [DOI] [PMC free article] [PubMed] [Google Scholar]