Abstract
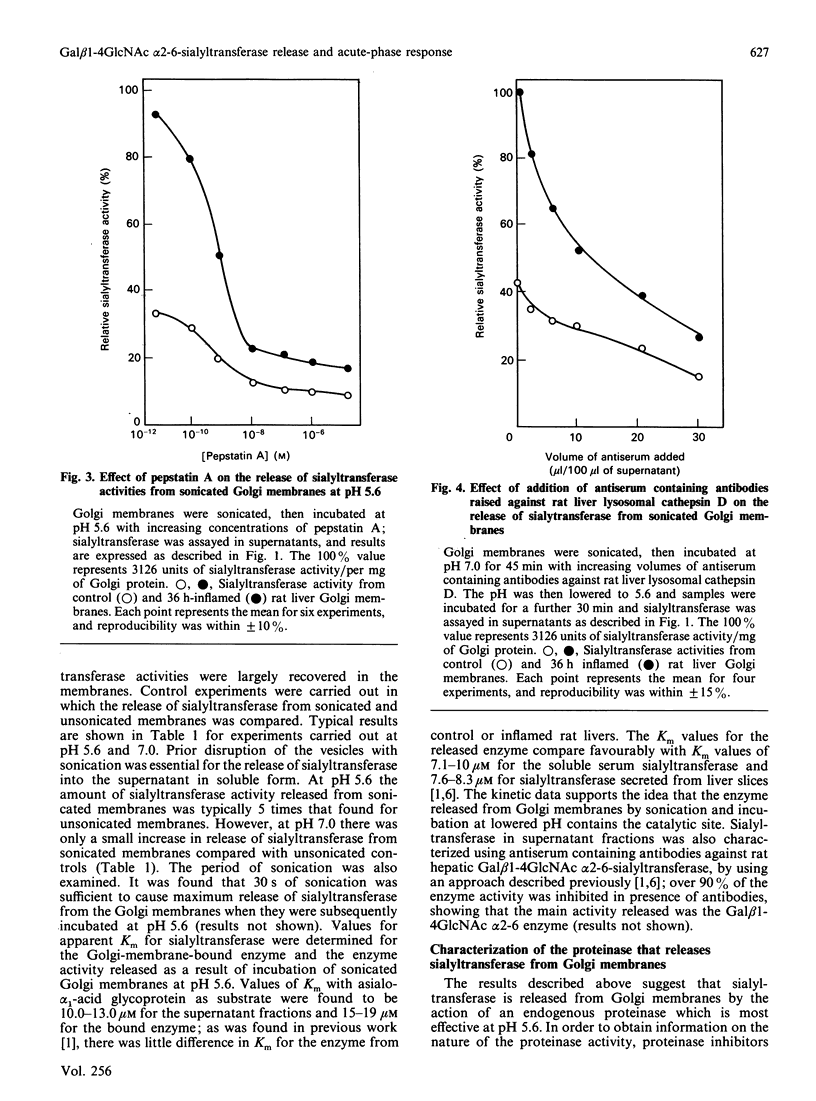
Golgi-membrane-bound Gal beta 1-4GlcNAc alpha 2-6-sialyltransferase (CMP-N-acetylneuraminate:beta-galactoside alpha 2-6-sialyltransferase, EC 2.4.99.1) behaves as an acute-phase reactant increasing about 5-fold in serum in rats suffering from inflammation. The mechanism of release from the Golgi membrane is not understood. In the present study it was found that sialyltransferase could be released from the membrane by treatment with ultrasonic vibration (sonication) followed by incubation at reduced pH. Maximum release occurred at pH 5.6, and membranes from inflamed rats released more enzyme than did membranes from controls. Galactosyltransferase (UDP-galactose:N-acetylglucosamine galactosyltransferase; EC 2.4.1.38), another Golgi-located enzyme, which does not behave as an acute-phase reactant, remained bound to the membranes under the same conditions. Release of the alpha 2-6-sialyltransferase from Golgi membranes was substantially inhibited by pepstatin A, a potent inhibitor of cathepsin D-like proteinases. Inhibition of release of the sialyltransferase also occurred after preincubation of sonicated Golgi membranes with antiserum raised against rat liver lysosomal cathepsin D. Addition of bovine spleen cathepsin D to incubation mixtures of sonicated Golgi membranes caused enhanced release of the sialyltransferase. Intact Golgi membranes were incubated at lowered pH in presence of pepstatin A to inhibit any proteinase activity at the cytosolic face; subsequent sonication showed that the sialyltransferase had been released, suggesting that the proteinase was active at the luminal face of the Golgi. Golgi membranes contained a low level of cathepsin D activity (EC 3.4.23.5); the enzyme was mainly membrane-bound, since it could only be released by extraction with Triton X-100 or incubation of sonicated Golgi membranes with 5 mM-mannose 6-phosphate. Immunoblot analysis showed that the transferase released from sonicated Golgi membranes at lowered pH had an apparent Mr of about 42,000 compared with one of about 49,000 for the membrane-bound enzyme. Values of Km for the bound and released enzyme activities were comparable and were similar to values reported previously for liver and serum enzymes. The work suggests that a major portion of sialyltransferase containing the catalytic site is released from a membrane anchor by a cathepsin D-like proteinase located at the luminal face of the Golgi and that this explains the acute-phase behaviour of this enzyme.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. G., Pathak R. K. Vesicles and cisternae in the trans Golgi apparatus of human fibroblasts are acidic compartments. Cell. 1985 Mar;40(3):635–643. doi: 10.1016/0092-8674(85)90212-0. [DOI] [PubMed] [Google Scholar]
- Ashton F. E., Jamieson J. C., Friesen A. D. Studies on the effect of inflammation on rat serum proteins. Can J Biochem. 1970 Aug;48(8):841–850. doi: 10.1139/o70-133. [DOI] [PubMed] [Google Scholar]
- Baricos W. H., Shah S. V. Increased cathepsin D-like activity in cortex, tubules, and glomeruli isolated from rats with experimental nephrotic syndrome. Biochem J. 1984 Oct 15;223(2):393–399. doi: 10.1042/bj2230393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J. Cathepsin D. Purification of isoenzymes from human and chicken liver. Biochem J. 1970 Apr;117(3):601–607. doi: 10.1042/bj1170601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisel W. R. Effects of infection on nutritional status and immunity. Fed Proc. 1980 Nov;39(13):3105–3108. [PubMed] [Google Scholar]
- Caplan M. J., Stow J. L., Newman A. P., Madri J., Anderson H. C., Farquhar M. G., Palade G. E., Jamieson J. D. Dependence on pH of polarized sorting of secreted proteins. Nature. 1987 Oct 15;329(6140):632–635. doi: 10.1038/329632a0. [DOI] [PubMed] [Google Scholar]
- Davidson H. W., Peshavaria M., Hutton J. C. Proteolytic conversion of proinsulin into insulin. Identification of a Ca2+-dependent acidic endopeptidase in isolated insulin-secretory granules. Biochem J. 1987 Sep 1;246(2):279–286. doi: 10.1042/bj2460279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean R. T. Lysosomes and protein degradation. Acta Biol Med Ger. 1977;36(11-12):1815–1820. [PubMed] [Google Scholar]
- Diment S., Leech M. S., Stahl P. D. Cathepsin D is membrane-associated in macrophage endosomes. J Biol Chem. 1988 May 15;263(14):6901–6907. [PubMed] [Google Scholar]
- Glickman J., Croen K., Kelly S., Al-Awqati Q. Golgi membranes contain an electrogenic H+ pump in parallel to a chloride conductance. J Cell Biol. 1983 Oct;97(4):1303–1308. doi: 10.1083/jcb.97.4.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G., Simons K. The trans Golgi network: sorting at the exit site of the Golgi complex. Science. 1986 Oct 24;234(4775):438–443. doi: 10.1126/science.2945253. [DOI] [PubMed] [Google Scholar]
- Haas A. L., Bright P. M. The immunochemical detection and quantitation of intracellular ubiquitin-protein conjugates. J Biol Chem. 1985 Oct 15;260(23):12464–12473. [PubMed] [Google Scholar]
- Jamieson J. C., Friesen A. D., Ashton F. E., Chou B. Studies on acute phase proteins of rat serum. 1. Isolation and partial characterization of an 1 -acid glycoprotein and an 2 -macroglobulin. Can J Biochem. 1972 Aug;50(8):856–870. doi: 10.1139/o72-121. [DOI] [PubMed] [Google Scholar]
- Jamieson J. C., Kaplan H. A., Woloski B. M., Hellman M., Ham K. Glycoprotein biosynthesis during the acute-phase response to inflammation. Can J Biochem Cell Biol. 1983 Sep;61(9):1041–1048. doi: 10.1139/o83-133. [DOI] [PubMed] [Google Scholar]
- Jamieson J. C., Lammers G., Janzen R., Woloski B. M. The acute phase response to inflammation: the role of monokines in changes in liver glycoproteins and enzymes of glycoprotein metabolism. Comp Biochem Physiol B. 1987;87(1):11–15. doi: 10.1016/0305-0491(87)90463-9. [DOI] [PubMed] [Google Scholar]
- Kaplan H. A., Woloski B. M., Hellman M., Jamieson J. C. Studies on the effect of inflammation on rat liver and serum sialyltransferase. Evidence that inflammation causes release of Gal beta 1 leads to 4GlcNAc alpha 2 leads to 6 sialyltransferase from liver. J Biol Chem. 1983 Oct 10;258(19):11505–11509. [PubMed] [Google Scholar]
- Kemmler W., Steiner D. F., Borg J. Studies on the conversion of proinsulin to insulin. 3. Studies in vitro with a crude secretion granule fraction isolated from rat islets of Langerhans. J Biol Chem. 1973 Jul 10;248(13):4544–4551. [PubMed] [Google Scholar]
- Kornfeld S. Trafficking of lysosomal enzymes. FASEB J. 1987 Dec;1(6):462–468. doi: 10.1096/fasebj.1.6.3315809. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lammers G., Jamieson J. C. Studies on the effect of experimental inflammation on sialyltransferase in the mouse and guinea pig. Comp Biochem Physiol B. 1986;84(2):181–187. doi: 10.1016/0305-0491(86)90203-8. [DOI] [PubMed] [Google Scholar]
- Moremen K. W., Touster O. Topology of mannosidase II in rat liver Golgi membranes and release of the catalytic domain by selective proteolysis. J Biol Chem. 1986 Aug 15;261(23):10945–10951. [PubMed] [Google Scholar]
- Paulson J. C., Weinstein J., Ujita E. L., Riggs K. J., Lai P. H. The membrane-binding domain of a rat liver Golgi sialyltransferase. Biochem Soc Trans. 1987 Aug;15(4):618–620. doi: 10.1042/bst0150618. [DOI] [PubMed] [Google Scholar]
- Quinn P. S., Judah J. D. Calcium-dependent Golgi-vesicle fusion and cathepsin B in the conversion of proalbumin into albumin in rat liver. Biochem J. 1978 May 15;172(2):301–309. doi: 10.1042/bj1720301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes C. J., Lucas C. A., Mutkoski R. L., Orci L., Halban P. A. Stimulation by ATP of proinsulin to insulin conversion in isolated rat pancreatic islet secretory granules. Association with the ATP-dependent proton pump. J Biol Chem. 1987 Aug 5;262(22):10712–10717. [PubMed] [Google Scholar]
- Roth J., Taatjes D. J., Lucocq J. M., Weinstein J., Paulson J. C. Demonstration of an extensive trans-tubular network continuous with the Golgi apparatus stack that may function in glycosylation. Cell. 1985 Nov;43(1):287–295. doi: 10.1016/0092-8674(85)90034-0. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Docherty K., Carroll R. Golgi/granule processing of peptide hormone and neuropeptide precursors: a minireview. J Cell Biochem. 1984;24(2):121–130. doi: 10.1002/jcb.240240204. [DOI] [PubMed] [Google Scholar]
- Taatjes D. J., Roth J., Weinstein J., Paulson J. C., Shaper N. L., Shaper J. H. Codistribution of galactosyl- and sialyltransferase: reorganization of trans Golgi apparatus elements in hepatocytes in intact liver and cell culture. Eur J Cell Biol. 1987 Oct;44(2):187–194. [PubMed] [Google Scholar]
- Weinstein J., Lee E. U., McEntee K., Lai P. H., Paulson J. C. Primary structure of beta-galactoside alpha 2,6-sialyltransferase. Conversion of membrane-bound enzyme to soluble forms by cleavage of the NH2-terminal signal anchor. J Biol Chem. 1987 Dec 25;262(36):17735–17743. [PubMed] [Google Scholar]
- Weinstein J., de Souza-e-Silva U., Paulson J. C. Purification of a Gal beta 1 to 4GlcNAc alpha 2 to 6 sialyltransferase and a Gal beta 1 to 3(4)GlcNAc alpha 2 to 3 sialyltransferase to homogeneity from rat liver. J Biol Chem. 1982 Nov 25;257(22):13835–13844. [PubMed] [Google Scholar]
- Weinstein J., de Souza-e-Silva U., Paulson J. C. Sialylation of glycoprotein oligosaccharides N-linked to asparagine. Enzymatic characterization of a Gal beta 1 to 3(4)GlcNAc alpha 2 to 3 sialyltransferase and a Gal beta 1 to 4GlcNAc alpha 2 to 6 sialyltransferase from rat liver. J Biol Chem. 1982 Nov 25;257(22):13845–13853. [PubMed] [Google Scholar]
- Woloski B. M., Fuller G. M., Jamieson J. C., Gospodarek E. Studies on the effect of the hepatocyte-stimulating factor on galactose-beta 1----4-N-acetylglucosamine alpha 2----6-sialyltransferase in cultured hepatocytes. Biochim Biophys Acta. 1986 Feb 21;885(2):185–191. doi: 10.1016/0167-4889(86)90087-x. [DOI] [PubMed] [Google Scholar]
- Woloski B. M., Gospodarek E., Jamieson J. C. Studies of monokines as mediators of the acute phase response: effects on sialyltransferase, albumin, alpha 1-acid glycoprotein and beta-N-acetylhexosaminidase. Biochem Biophys Res Commun. 1985 Jul 16;130(1):30–36. doi: 10.1016/0006-291x(85)90377-8. [DOI] [PubMed] [Google Scholar]
- Woloski B. M., Kaplan H. A., Gospodarek E., Jamieson J. C. Studies on rat cytokines as mediators of the acute phase response. Biochem Biophys Res Commun. 1983 Apr 15;112(1):14–19. doi: 10.1016/0006-291x(83)91790-4. [DOI] [PubMed] [Google Scholar]



