Abstract
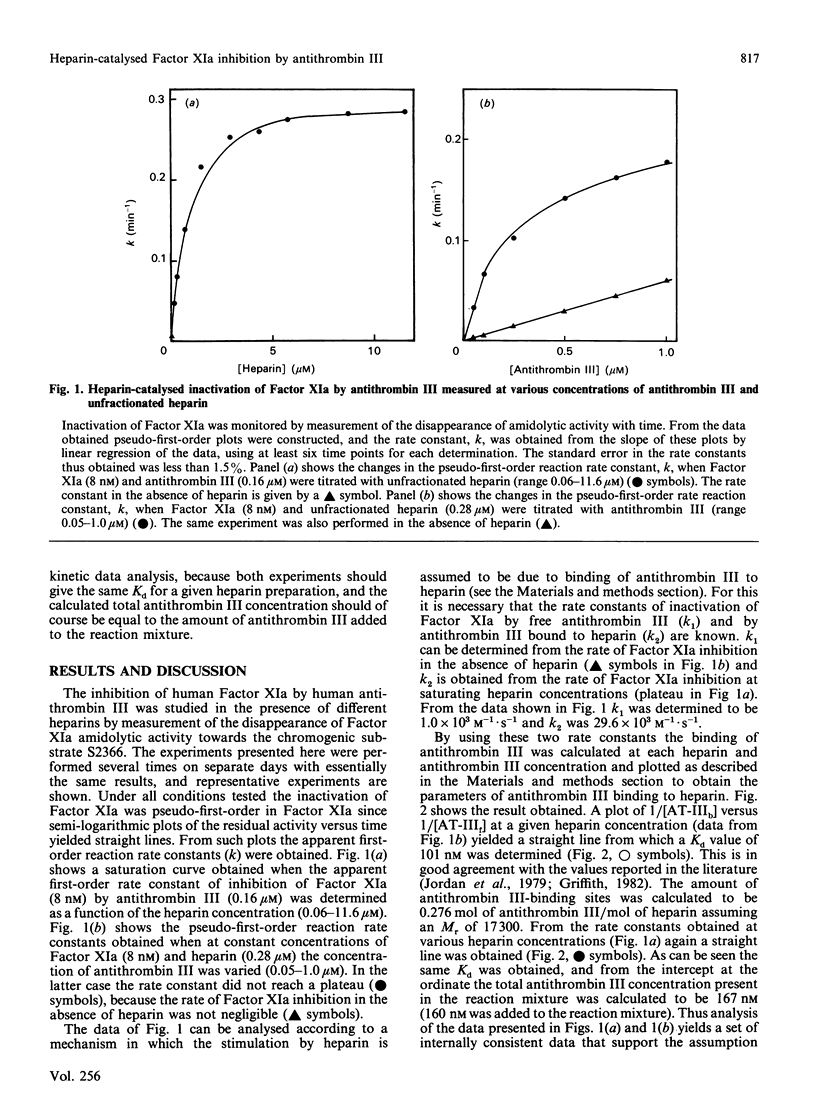
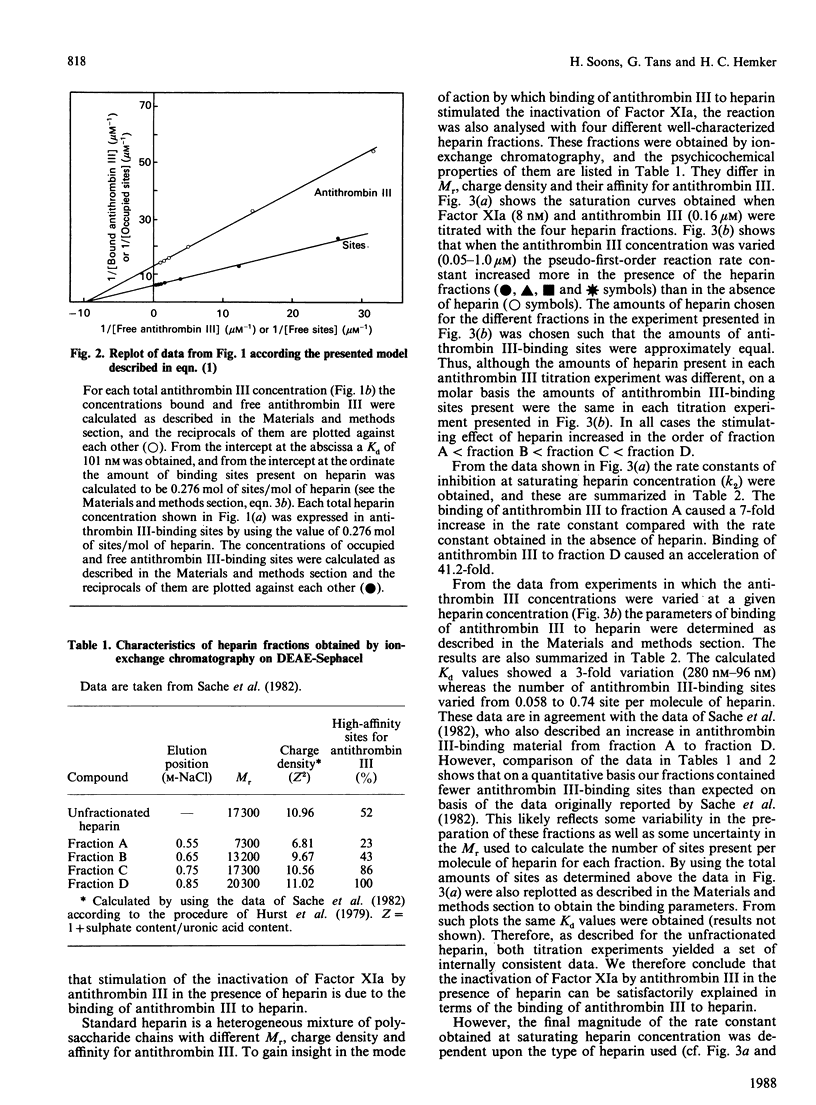
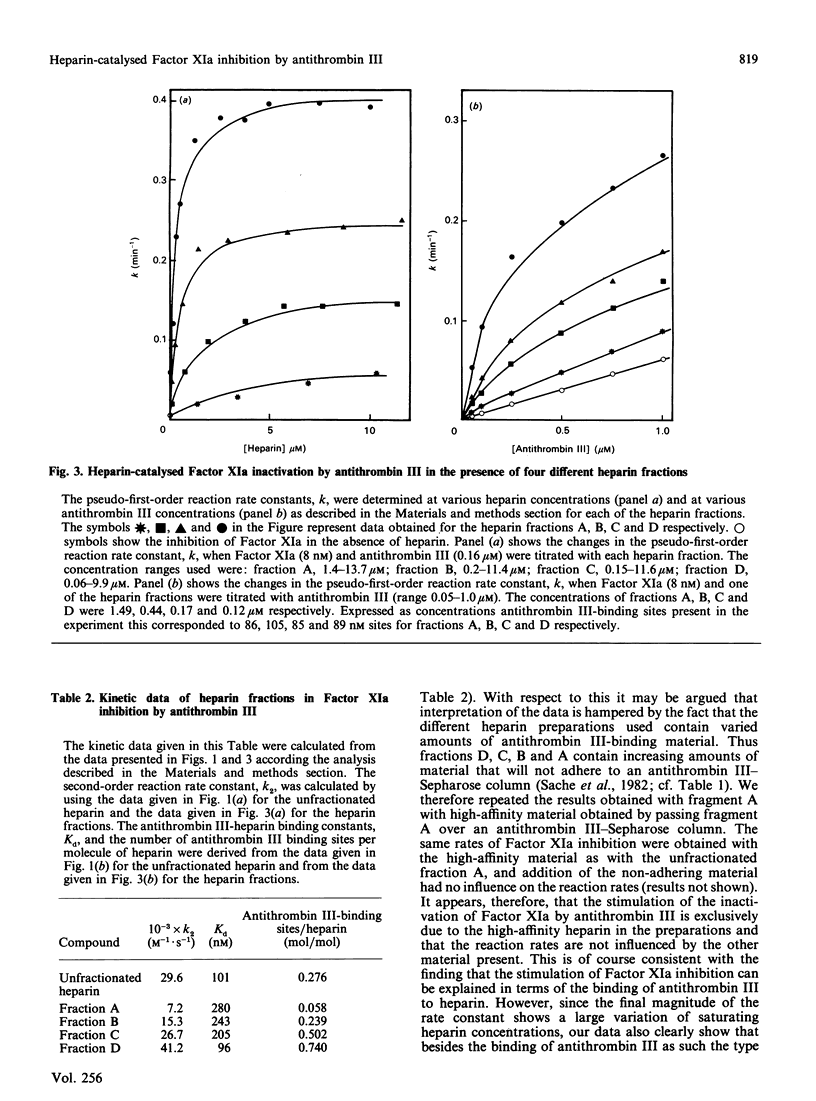
The effect of various well-characterized heparin preparations on the inactivation of human Factor XIa by human antithrombin III was studied. The heparin preparations used were unfractionated heparin and four heparin fractions obtained after anion-exchange chromatography. Inactivation of Factor XIa was monitored with S2366 as chromogenic substrate and followed pseudo-first-order reaction kinetics under all reaction conditions tested. Enhancement of the rate of inhibition of Factor XIa in the presence of unfractionated heparin correlated to the binding of antithrombin III to heparin. From the kinetic data a binding constant of 0.1 microM was inferred. The maximum rate enhancement, achieved at saturating heparin concentrations, was 30-fold. The rate enhancement achieved in the presence of each of the heparin fractions could also be correlated to the binding of antithrombin III to the heparin. The binding constant inferred from the kinetic data varied from 0.10 to 0.28 microM and the number of binding sites for antithrombin III varied from 0.06 to 0.74 site per heparin molecule. The maximum rate enhancements, achieved at saturating heparin concentrations, were strongly dependent on the type of heparin used and varied from 7-fold for fraction A to 41-fold for fraction D. Therefore, although the stimulation of Factor XIa inactivation by antithrombin III could be quantitatively correlated to the binding of antithrombin III to heparin, the heparin-catalysed inhibition of Factor XIa is dependent not only upon the degree of binding of antithrombin III to heparin but also upon the type of heparin to which antithrombin III is bound.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beeler D. L., Marcum J. A., Schiffman S., Rosenberg R. D. Interaction of factor XIa and antithrombin in the presence and absence of heparin. Blood. 1986 May;67(5):1488–1492. [PubMed] [Google Scholar]
- Beeler D., Rosenberg R., Jordan R. Fractionation of low molecular weight heparin species and their interaction with antithrombin. J Biol Chem. 1979 Apr 25;254(8):2902–2913. [PubMed] [Google Scholar]
- Bouma B. N., Griffin J. H. Human blood coagulation factor XI. Purification, properties, and mechanism of activation by activated factor XII. J Biol Chem. 1977 Sep 25;252(18):6432–6437. [PubMed] [Google Scholar]
- Bouma B. N., Vlooswijk R. A., Griffin J. H. Immunologic studies of human coagulation factor XI and its complex with high molecular weight kininogen. Blood. 1983 Nov;62(5):1123–1131. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Fujikawa K., Chung D. W., Hendrickson L. E., Davie E. W. Amino acid sequence of human factor XI, a blood coagulation factor with four tandem repeats that are highly homologous with plasma prekallikrein. Biochemistry. 1986 May 6;25(9):2417–2424. doi: 10.1021/bi00357a018. [DOI] [PubMed] [Google Scholar]
- Fujikawa K., McMullen B. A. Amino acid sequence of human beta-factor XIIa. J Biol Chem. 1983 Sep 25;258(18):10924–10933. [PubMed] [Google Scholar]
- Griffin J. H., Cochrane C. G. Human factor XII (Hageman factor). Methods Enzymol. 1976;45:56–65. doi: 10.1016/s0076-6879(76)45009-7. [DOI] [PubMed] [Google Scholar]
- Griffith M. J. Kinetics of the heparin-enhanced antithrombin III/thrombin reaction. Evidence for a template model for the mechanism of action of heparin. J Biol Chem. 1982 Jul 10;257(13):7360–7365. [PubMed] [Google Scholar]
- Holmer E., Kurachi K., Söderström G. The molecular-weight dependence of the rate-enhancing effect of heparin on the inhibition of thrombin, factor Xa, factor IXa, factor XIa, factor XIIa and kallikrein by antithrombin. Biochem J. 1981 Feb 1;193(2):395–400. doi: 10.1042/bj1930395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst R. E., Menter J. M., West S. S., Settine J. M., Coyne E. H. Structural basis for the anticoagulant activity of heparin. 1. Relationship to the number of charged groups. Biochemistry. 1979 Oct 2;18(20):4283–4287. doi: 10.1021/bi00587a004. [DOI] [PubMed] [Google Scholar]
- Kurachi K., Davie E. W. Activation of human factor XI (plasma thromboplastin antecedent) by factor XIIa (activated Hageman factor). Biochemistry. 1977 Dec 27;16(26):5831–5839. doi: 10.1021/bi00645a030. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nesheim M. E. A simple rate law that describes the kinetics of the heparin-catalyzed reaction between antithrombin III and thrombin. J Biol Chem. 1983 Dec 10;258(23):14708–14717. [PubMed] [Google Scholar]
- Osterud B., Rapaport S. I. Activation of factor IX by the reaction product of tissue factor and factor VII: additional pathway for initiating blood coagulation. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5260–5264. doi: 10.1073/pnas.74.12.5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sache E., Maillard M., Bertrand H., Maman M., Kunz M., Choay J., Fareed J., Messmore H. Studies on a highly active anticoagulant fraction of high molecular weight isolated from porcine sodium heparin. Thromb Res. 1982 Mar 15;25(6):443–458. doi: 10.1016/0049-3848(82)90086-x. [DOI] [PubMed] [Google Scholar]
- Scott C. F., Schapira M., James H. L., Cohen A. B., Colman R. W. Inactivation of factor XIa by plasma protease inhibitors: predominant role of alpha 1-protease inhibitor and protective effect of high molecular weight kininogen. J Clin Invest. 1982 Apr;69(4):844–852. doi: 10.1172/JCI110524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soons H., Janssen-Claessen T., Hemker H. C., Tans G. The effect of platelets in the activation of human blood coagulation factor IX by factor XIa. Blood. 1986 Jul;68(1):140–148. [PubMed] [Google Scholar]
- Soons H., Janssen-Claessen T., Tans G., Hemker H. C. Inhibition of factor XIa by antithrombin III. Biochemistry. 1987 Jul 28;26(15):4624–4629. doi: 10.1021/bi00389a005. [DOI] [PubMed] [Google Scholar]
- Thaler E., Schmer G. A simple two-step isolation procedure for human and bovine antithrombin II/III (heparin cofactor): a comparison of two methods. Br J Haematol. 1975 Oct;31(2):233–243. doi: 10.1111/j.1365-2141.1975.tb00853.x. [DOI] [PubMed] [Google Scholar]
- van der Graaf F., Greengard J. S., Bouma B. N., Kerbiriou D. M., Griffin J. H. Isolation and functional characterization of the active light chain of activated human blood coagulation factor XI. J Biol Chem. 1983 Aug 25;258(16):9669–9675. [PubMed] [Google Scholar]


