Abstract
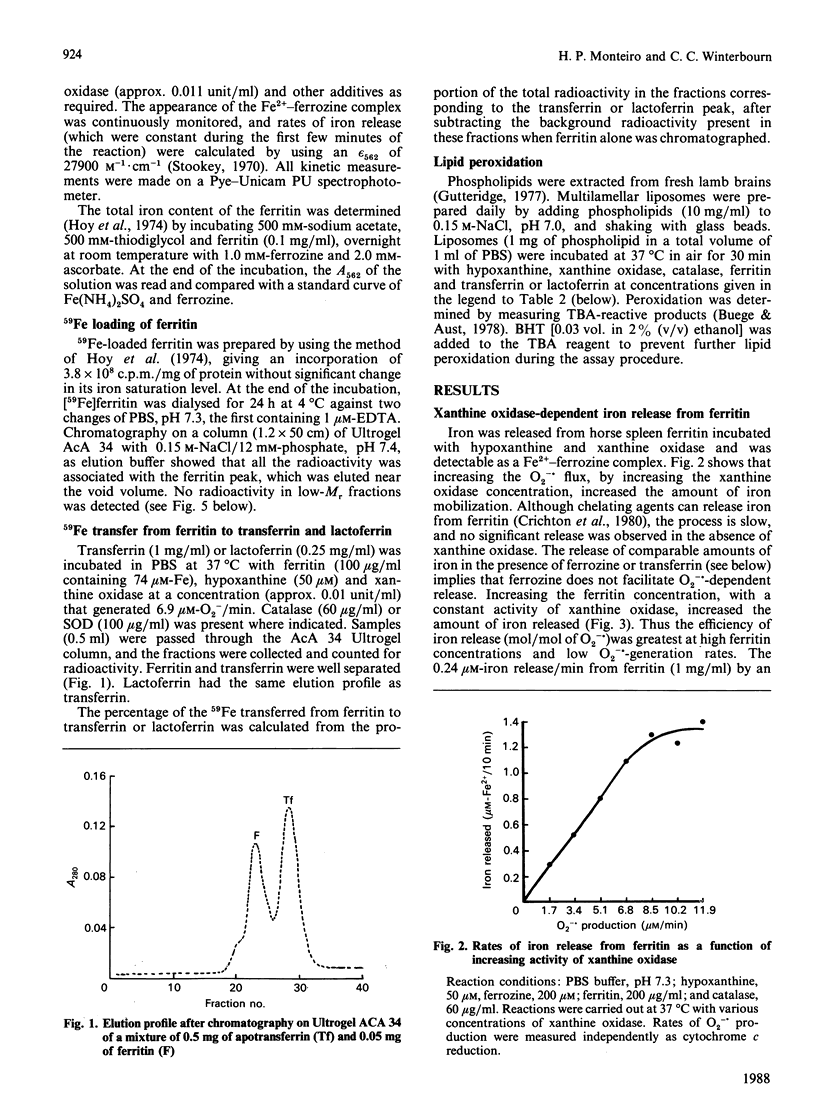
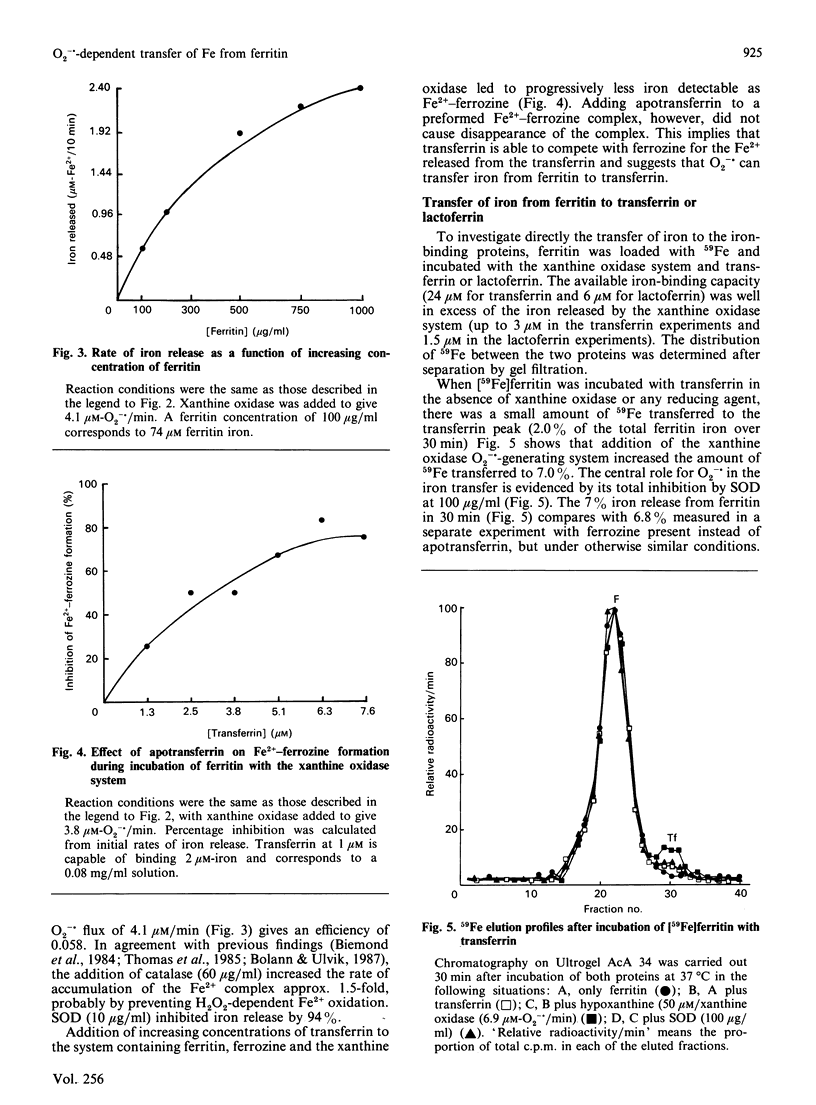
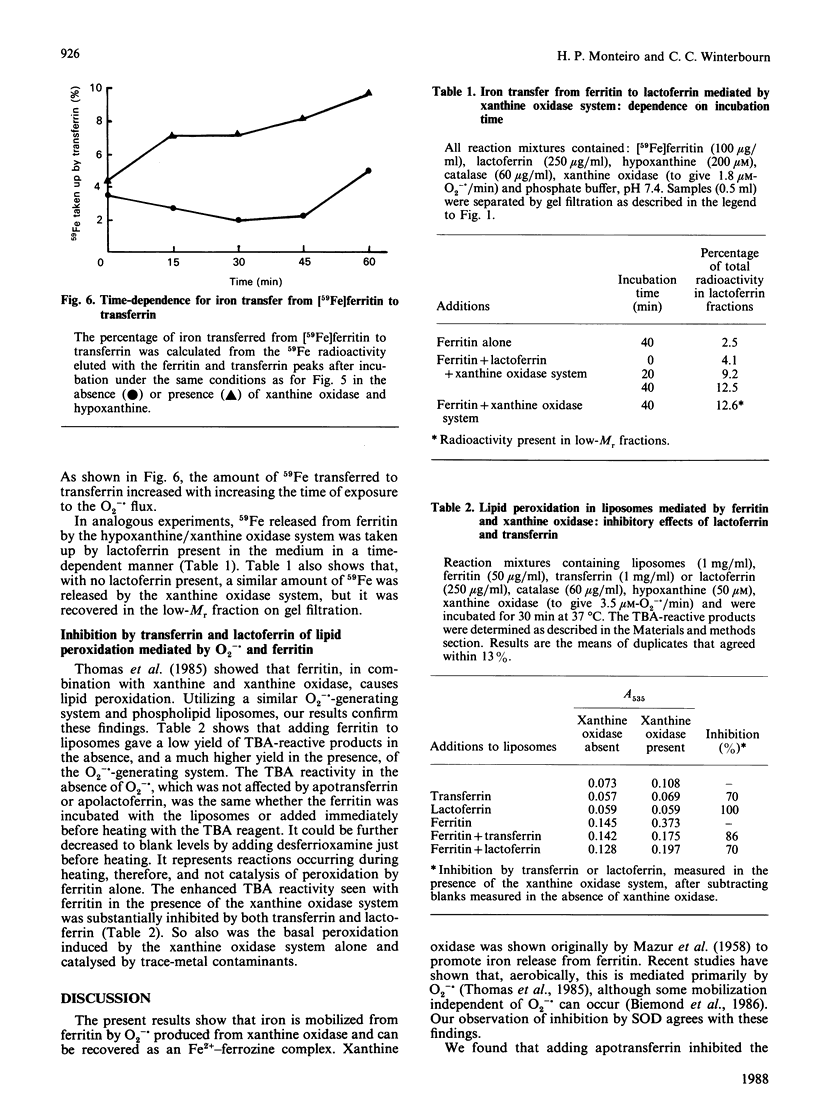
By the use of gel filtration and [59Fe]ferritin, apotransferrin and apolactoferrin were shown to take up iron released from ferritin by superoxide generated by hypoxanthine and xanthine oxidase. Apotransferrin also inhibited uptake of released iron by ferrozine. Ferritin and the xanthine oxidase system induced lipid peroxidation in phospholipid liposomes. This peroxidation was inhibited by apotransferrin or apolactoferrin. Thus, although superoxide and other free radicals can release iron from ferritin, either iron-binding protein, if present, should take up this iron and prevent its catalysing subsequent oxidative reactions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aruoma O. I., Halliwell B. Superoxide-dependent and ascorbate-dependent formation of hydroxyl radicals from hydrogen peroxide in the presence of iron. Are lactoferrin and transferrin promoters of hydroxyl-radical generation? Biochem J. 1987 Jan 1;241(1):273–278. doi: 10.1042/bj2410273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aust S. D., Morehouse L. A., Thomas C. E. Role of metals in oxygen radical reactions. J Free Radic Biol Med. 1985;1(1):3–25. doi: 10.1016/0748-5514(85)90025-x. [DOI] [PubMed] [Google Scholar]
- Baldwin D. A., Jenny E. R., Aisen P. The effect of human serum transferrin and milk lactoferrin on hydroxyl radical formation from superoxide and hydrogen peroxide. J Biol Chem. 1984 Nov 10;259(21):13391–13394. [PubMed] [Google Scholar]
- Biemond P., Swaak A. J., Beindorff C. M., Koster J. F. Superoxide-dependent and -independent mechanisms of iron mobilization from ferritin by xanthine oxidase. Implications for oxygen-free-radical-induced tissue destruction during ischaemia and inflammation. Biochem J. 1986 Oct 1;239(1):169–173. doi: 10.1042/bj2390169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biemond P., van Eijk H. G., Swaak A. J., Koster J. F. Iron mobilization from ferritin by superoxide derived from stimulated polymorphonuclear leukocytes. Possible mechanism in inflammation diseases. J Clin Invest. 1984 Jun;73(6):1576–1579. doi: 10.1172/JCI111364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolann B. J., Ulvik R. J. Release of iron from ferritin by xanthine oxidase. Role of the superoxide radical. Biochem J. 1987 Apr 1;243(1):55–59. doi: 10.1042/bj2430055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer R. F., McCleary C. J. Superoxide ion as a primary reductant in ascorbate-mediated ferritin iron release. Free Radic Biol Med. 1987;3(6):389–395. doi: 10.1016/0891-5849(87)90017-7. [DOI] [PubMed] [Google Scholar]
- Britigan B. E., Rosen G. M., Chai Y., Cohen M. S. Do human neutrophils make hydroxyl radical? Determination of free radicals generated by human neutrophils activated with a soluble or particulate stimulus using electron paramagnetic resonance spectrometry. J Biol Chem. 1986 Apr 5;261(10):4426–4431. [PubMed] [Google Scholar]
- Buege J. A., Aust S. D. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- Crichton R. R., Roman F., Roland F. Ferritin iron mobilisation by chelating agents. FEBS Lett. 1980 Feb 11;110(2):271–274. doi: 10.1016/0014-5793(80)80090-1. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Halliwell B., Treffry A., Harrison P. M., Blake D. Effect of ferritin-containing fractions with different iron loading on lipid peroxidation. Biochem J. 1983 Feb 1;209(2):557–560. doi: 10.1042/bj2090557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M., Paterson S. K., Segal A. W., Halliwell B. Inhibition of lipid peroxidation by the iron-binding protein lactoferrin. Biochem J. 1981 Oct 1;199(1):259–261. doi: 10.1042/bj1990259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M. The measurement of malondialdehyde in peroxidised ox-brain phospholipid liposomes. Anal Biochem. 1977 Sep;82(1):76–82. doi: 10.1016/0003-2697(77)90136-1. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen free radicals and iron in relation to biology and medicine: some problems and concepts. Arch Biochem Biophys. 1986 May 1;246(2):501–514. doi: 10.1016/0003-9861(86)90305-x. [DOI] [PubMed] [Google Scholar]
- Hoy T. G., Harrison P. M., Shabbir M. Uptake and release of ferritin iron. Surface effects and exchange within the crystalline core. Biochem J. 1974 Jun;139(3):603–607. doi: 10.1042/bj1390603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Crichton R. R. Iron transfer from ferritin to transferrin. Effect of serum factors. FEBS Lett. 1987 May 4;215(1):41–46. doi: 10.1016/0014-5793(87)80110-2. [DOI] [PubMed] [Google Scholar]
- Kontoghiorghes G. J. 2-Hydroxypyridine-N-oxides: effective new chelators in iron mobilisation. Biochim Biophys Acta. 1987 Apr 16;924(1):13–18. doi: 10.1016/0304-4165(87)90065-1. [DOI] [PubMed] [Google Scholar]
- Koster J. F., Slee R. G. Ferritin, a physiological iron donor for microsomal lipid peroxidation. FEBS Lett. 1986 Apr 7;199(1):85–88. doi: 10.1016/0014-5793(86)81228-5. [DOI] [PubMed] [Google Scholar]
- MAZUR A., GREEN S., SAHA A., CARLETON A. Mechanism of release of ferritin iron in vivo by xanthine oxidase. J Clin Invest. 1958 Dec;37(12):1809–1817. doi: 10.1172/JCI103774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Muirden K. D. Ferritin in synovial cells in patients with rheumatoid arthritis. Ann Rheum Dis. 1966 Sep;25(5):387–401. doi: 10.1136/ard.25.5.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muirden K. D., Senator G. B. Iron in the synovial membrane in rheumatoid arthritis and other joint diseases. Ann Rheum Dis. 1968 Jan;27(1):38–48. doi: 10.1136/ard.27.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell M. J., Ward R. J., Baum H., Peters T. J. The role of iron in ferritin- and haemosiderin-mediated lipid peroxidation in liposomes. Biochem J. 1985 Jul 1;229(1):135–139. doi: 10.1042/bj2290135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K., Nishisato T., Grasso J. A., Aisen P. Interaction of transferrin with iron-loaded rat peritoneal macrophages. Br J Haematol. 1986 Feb;62(2):275–286. doi: 10.1111/j.1365-2141.1986.tb02930.x. [DOI] [PubMed] [Google Scholar]
- Theil E. C. Ferritin: structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu Rev Biochem. 1987;56:289–315. doi: 10.1146/annurev.bi.56.070187.001445. [DOI] [PubMed] [Google Scholar]
- Thomas C. E., Aust S. D. Reductive release of iron from ferritin by cation free radicals of paraquat and other bipyridyls. J Biol Chem. 1986 Oct 5;261(28):13064–13070. [PubMed] [Google Scholar]
- Thomas C. E., Aust S. D. Release of iron from ferritin by cardiotoxic anthracycline antibiotics. Arch Biochem Biophys. 1986 Aug 1;248(2):684–689. doi: 10.1016/0003-9861(86)90523-0. [DOI] [PubMed] [Google Scholar]
- Thomas C. E., Morehouse L. A., Aust S. D. Ferritin and superoxide-dependent lipid peroxidation. J Biol Chem. 1985 Mar 25;260(6):3275–3280. [PubMed] [Google Scholar]
- Thomas M. J., Shirley P. S., Hedrick C. C., DeChatelet L. R. Role of free radical processes in stimulated human polymorphonuclear leukocytes. Biochemistry. 1986 Dec 2;25(24):8042–8048. doi: 10.1021/bi00372a037. [DOI] [PubMed] [Google Scholar]
- Watt G. D., Frankel R. B., Papaefthymiou G. C. Reduction of mammalian ferritin. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3640–3643. doi: 10.1073/pnas.82.11.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills E. D. Mechanisms of lipid peroxide formation in animal tissues. Biochem J. 1966 Jun;99(3):667–676. doi: 10.1042/bj0990667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willson R. L. Iron, zinc, free radicals and oxygen in tissue disorders and cancer control. Ciba Found Symp. 1976 Dec 7;(51):331–354. doi: 10.1002/9780470720325.ch16. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C. Lactoferrin-catalysed hydroxyl radical production. Additional requirement for a chelating agent. Biochem J. 1983 Jan 15;210(1):15–19. doi: 10.1042/bj2100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn C. C. Myeloperoxidase as an effective inhibitor of hydroxyl radical production. Implications for the oxidative reactions of neutrophils. J Clin Invest. 1986 Aug;78(2):545–550. doi: 10.1172/JCI112607. [DOI] [PMC free article] [PubMed] [Google Scholar]


