Abstract
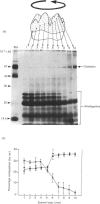
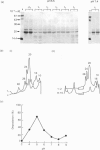
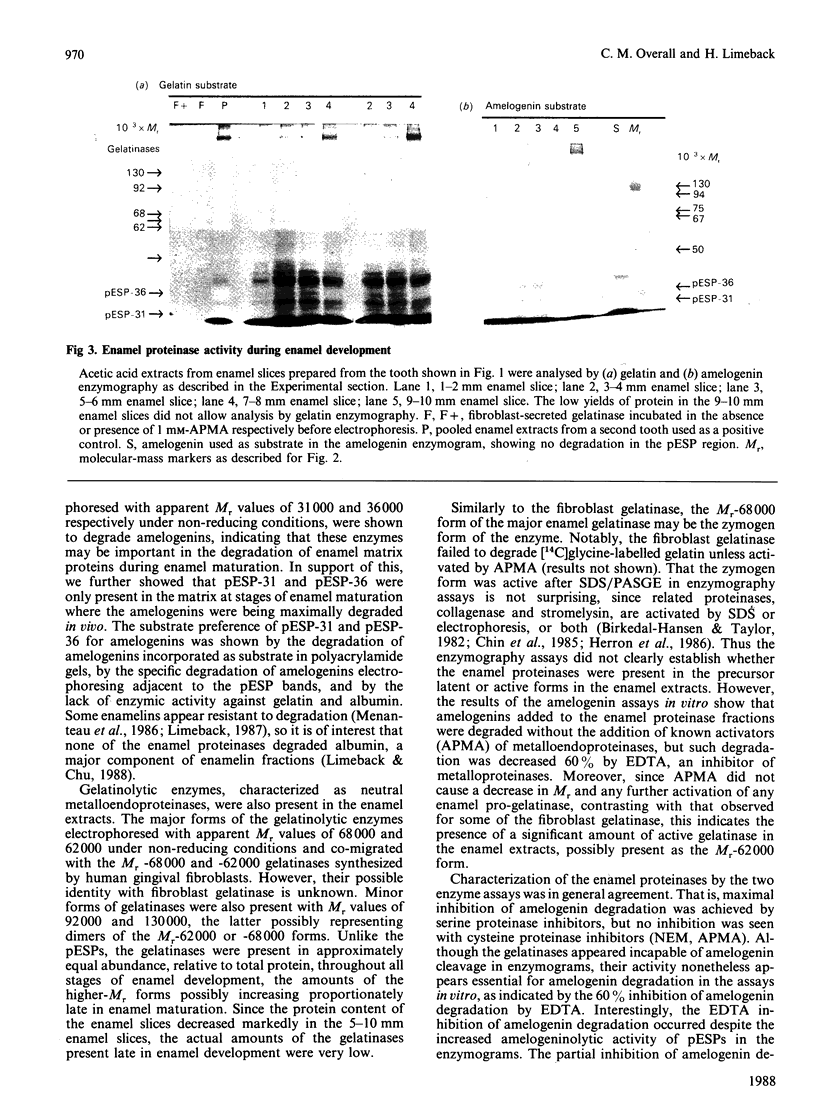
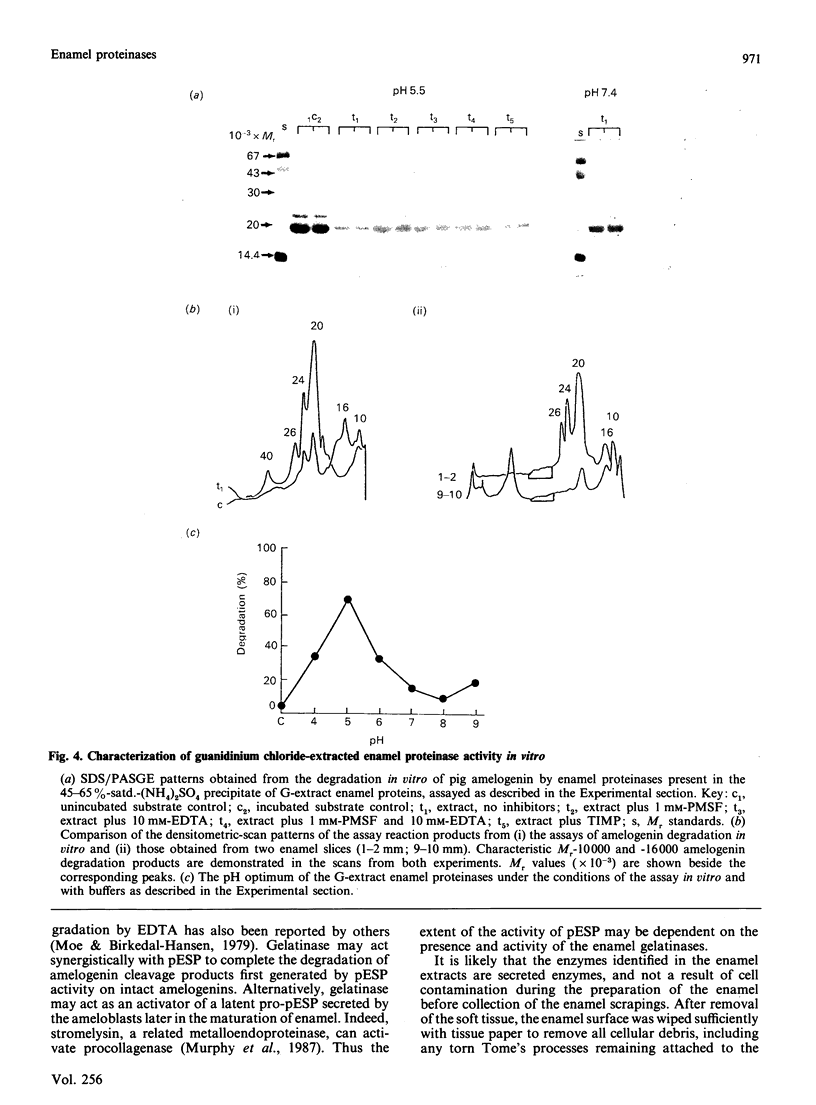
During tooth formation nearly all of the protein matrix of enamel is removed before final mineralization. To study this process, enamel proteins and proteinases were extracted from pig enamel at different stages of tooth development. In the enamel maturation zones, the major enamel matrix proteins, the amelogenins, were rapidly processed and removed. Possibly associated with this process in vivo are two groups of proteinases which were identified in the enamel extracts by enzymography using amelogenin-substrate and gelatin-substrate polyacrylamide gels and by the degradation in vitro of guanidinium chloride-extracted amelogenins. One group of proteinases with gelatinolytic activity consisted of several neutral metalloendoproteinases having Mr values from 62,000 to 130,000. These proteinases were inactive against amelogenins, casein and albumin, and were present in approximately equal proportions in enamel at all developmental stages. In the other group, two serine proteinases, with apparent non-reduced Mr of 31,000 and 36,000 exhibited amelogeninolytic activity. The substrate preference of the enamel serine proteinases was indicated by their limited degradation of casein and their inability to degrade gelatin and albumin. Contrasting with the distribution of the metalloendoproteinase enzymes, the serine proteinases were found only in the enamel scrapings taken from late-maturing enamel. The amelogenin degradation patterns in vivo, observed in the enamel scrapings, were similar to those produced in assays in vitro using partially purified fractions of enamel proteinases and amelogenin substrate. Together, these data strongly indicate an important role for the serine proteinases, and possibly the gelatinolytic proteinases, in the organized processing of the enamel protein matrix during enamel formation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birkedal-Hansen H., Taylor R. E. Detergent-activation of latent collagenase and resolution of its component molecules. Biochem Biophys Res Commun. 1982 Aug 31;107(4):1173–1178. doi: 10.1016/s0006-291x(82)80120-4. [DOI] [PubMed] [Google Scholar]
- Chin J. R., Murphy G., Werb Z. Stromelysin, a connective tissue-degrading metalloendopeptidase secreted by stimulated rabbit synovial fibroblasts in parallel with collagenase. Biosynthesis, isolation, characterization, and substrates. J Biol Chem. 1985 Oct 5;260(22):12367–12376. [PubMed] [Google Scholar]
- Herron G. S., Banda M. J., Clark E. J., Gavrilovic J., Werb Z. Secretion of metalloproteinases by stimulated capillary endothelial cells. II. Expression of collagenase and stromelysin activities is regulated by endogenous inhibitors. J Biol Chem. 1986 Feb 25;261(6):2814–2818. [PubMed] [Google Scholar]
- Heussen C., Dowdle E. B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980 Feb;102(1):196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Limeback H. Isolation and characterization of pig enamelins. Biochem J. 1987 Apr 15;243(2):385–390. doi: 10.1042/bj2430385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. T., Burgess D. R. SDS microslab linear gradient polyacrylamide gel electrophoresis. Anal Biochem. 1978 Jul 1;87(2):386–396. doi: 10.1016/0003-2697(78)90688-7. [DOI] [PubMed] [Google Scholar]
- Menanteau J., Mitre D., Raher S. An in-vitro study of enamel protein degradation in developing bovine enamel. Arch Oral Biol. 1986;31(12):807–810. doi: 10.1016/0003-9969(86)90132-9. [DOI] [PubMed] [Google Scholar]
- Moe D., Birkedal-Hansen H. Proteolytic activity in developing bovine enamel. J Dent Res. 1979 Mar;58(SPEC):1012–1013. doi: 10.1177/002203457905800207011. [DOI] [PubMed] [Google Scholar]
- Murphy G., Cockett M. I., Stephens P. E., Smith B. J., Docherty A. J. Stromelysin is an activator of procollagenase. A study with natural and recombinant enzymes. Biochem J. 1987 Nov 15;248(1):265–268. doi: 10.1042/bj2480265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall C. M., Sodek J. Initial characterization of a neutral metalloproteinase, active on native 3/4-collagen fragments, synthesized by ROS 17/2.8 osteoblastic cells, periodontal fibroblasts, and identified in gingival crevicular fluid. J Dent Res. 1987 Jul;66(7):1271–1282. doi: 10.1177/00220345870660071201. [DOI] [PubMed] [Google Scholar]
- Overall C. M., Wiebkin O. W., Thonard J. C. Demonstration of tissue collagenase activity in vivo and its relationship to inflammation severity in human gingiva. J Periodontal Res. 1987 Mar;22(2):81–88. doi: 10.1111/j.1600-0765.1987.tb01544.x. [DOI] [PubMed] [Google Scholar]
- Robinson C., Kirkham J., Briggs H. D., Atkinson P. J. Enamel proteins: from secretion to maturation. J Dent Res. 1982 Dec;Spec No:1490–1495. [PubMed] [Google Scholar]
- Robinson C., Kirkham J., Weatherell J. A., Richards A., Josephsen K., Fejerskov O. Developmental stages in permanent porcine enamel. Acta Anat (Basel) 1987;128(1):1–10. doi: 10.1159/000146306. [DOI] [PubMed] [Google Scholar]
- Robinson R. M., Taylor R. E., Birkedal-Hansen H. Evidence for an extracellular plasmin-dependent proteolytic system in mineralizing matrices. Calcif Tissue Int. 1984 Jan;36(1):31–38. doi: 10.1007/BF02405291. [DOI] [PubMed] [Google Scholar]
- Shimizu M., Tanabe T., Fukae M. Proteolytic enzyme in porcine immature enamel. J Dent Res. 1979 Mar;58(SPEC):782–789. doi: 10.1177/00220345790580023001. [DOI] [PubMed] [Google Scholar]
- Slavkin H. C., Weliky B., Stellar W., Slavkin M. D., Zeichner-Gancz M., Bringas P., Jr, Hyatt-Fischer H., Shimizu M., Fukae M. Ameloblast differentiation: protein synthesis and secretion in fetal New Zealand white rabbit molar tooth organs and isolated epithelia "in vitro". J Biol Buccale. 1978 Dec;6(4):309–326. [PubMed] [Google Scholar]
- Termine J. D., Belcourt A. B., Christner P. J., Conn K. M., Nylen M. U. Properties of dissociatively extracted fetal tooth matrix proteins. I. Principal molecular species in developing bovine enamel. J Biol Chem. 1980 Oct 25;255(20):9760–9768. [PubMed] [Google Scholar]