Abstract
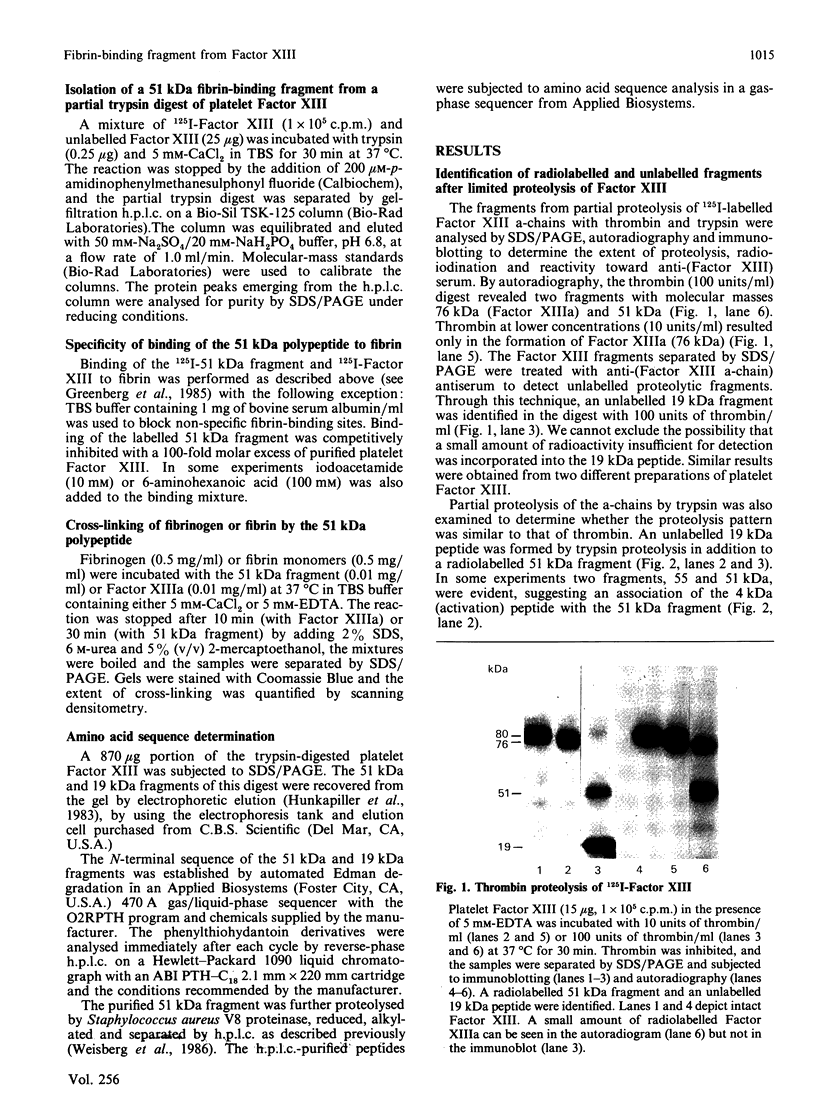
Purified platelet Factor XIII was radioiodinated and then partially degraded by thrombin or trypsin, and a fibrin-binding fragment was identified by autoradiography and immunoblotting following separation by SDS/polyacrylamide-gel electrophoresis. Limited proteolysis of 125I-Factor XIII by thrombin or trypsin produced an 125I-51 kDa fragment and an unlabelled 19 kDa fragment. The 51 kDa fragment was purified by h.p.l.c. on a TSK-125 gel-filtration column. Partial amino acid sequence analysis of the 51 kDa fragment indicated that it was similar in sequence to the Gly38-Lys513 segment in placental Factor XIII a-chain. More than 70% of the 51 kDa fragment bound to fibrin, whereas the 19 kDa fragment did not bind. The active site was localized to the 51 kDa fragment since this fragment expressed transglutaminase activity, cross-linked fibrin and fibrinogen and incorporated iodo[14C]acetamide into the active-site cysteine residue. Isolation of a fibrin-binding fragment expressing transglutaminase activity demonstrates that each a-chain of the dimeric Factor XIIIa could function independently to cross-link fibrin. The fibrin-binding site could play an important role in localizing Factor XIIIa to the fibrin clot.
Full text
PDF

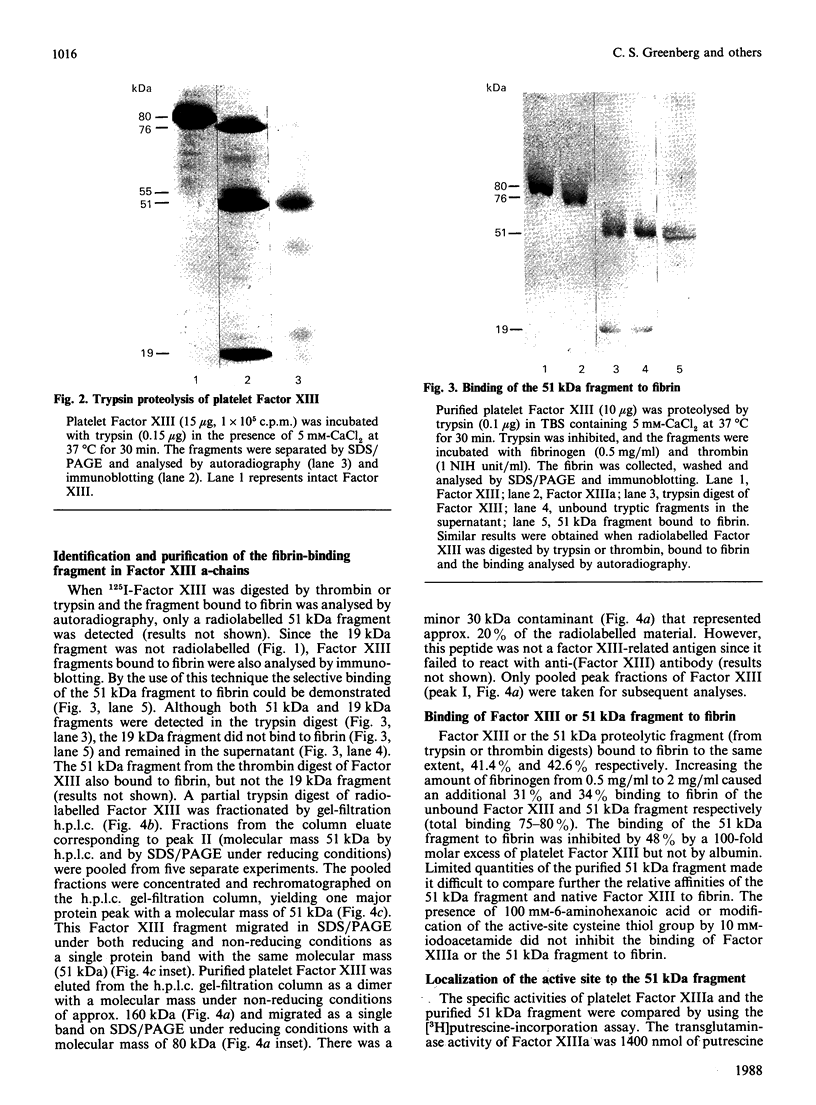



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chung S. I., Lewis M. S., Folk J. E. Relationships of the catalytic properties of human plasma and platelet transglutaminases (activated blood coagulation factor XIII) to their subunit structures. J Biol Chem. 1974 Feb 10;249(3):940–950. [PubMed] [Google Scholar]
- Credo R. B., Curtis C. G., Lorand L. Alpha-chain domain of fibrinogen controls generation of fibrinoligase (coagulation factor XIIIa). Calcium ion regulatory aspects. Biochemistry. 1981 Jun 23;20(13):3770–3778. doi: 10.1021/bi00516a016. [DOI] [PubMed] [Google Scholar]
- Credo R. B., Curtis C. G., Lorand L. Ca2+-related regulatory function of fibrinogen. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4234–4237. doi: 10.1073/pnas.75.9.4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis C. G., Brown K. L., Credo R. B., Domanik R. A., Gray A., Stenberg P., Lorand L. Calcium-dependent unmasking of active center cysteine during activation of fibrin stabilizing factor. Biochemistry. 1974 Aug 27;13(18):3774–3780. doi: 10.1021/bi00715a024. [DOI] [PubMed] [Google Scholar]
- Dvilansky A., Britten A. F., Loewy A. G. Factor XIII assay by an isotope method. I. Factor XIII (transamidase) in plasma, serum, leucocytes, erythrocytes and platelets and evaluation of screening tests of clot solubility. Br J Haematol. 1970 Apr;18(4):399–410. doi: 10.1111/j.1365-2141.1970.tb01453.x. [DOI] [PubMed] [Google Scholar]
- Greenberg C. S., Achyuthan K. E., Fenton J. W., 2nd Factor XIIIa formation promoted by complexing of alpha-thrombin, fibrin, and plasma factor XIII. Blood. 1987 Mar;69(3):867–871. [PubMed] [Google Scholar]
- Greenberg C. S., Dobson J. V., Miraglia C. C. Regulation of plasma factor XIII binding to fibrin in vitro. Blood. 1985 Nov;66(5):1028–1034. [PubMed] [Google Scholar]
- Greenberg C. S., Miraglia C. C. The effect of fibrin polymers on thrombin-catalyzed plasma factor XIIIa formation. Blood. 1985 Aug;66(2):466–469. [PubMed] [Google Scholar]
- Greenberg C. S., Shuman M. A. The zymogen forms of blood coagulation factor XIII bind specifically to fibrinogen. J Biol Chem. 1982 Jun 10;257(11):6096–6101. [PubMed] [Google Scholar]
- Grundmann U., Amann E., Zettlmeissl G., Küpper H. A. Characterization of cDNA coding for human factor XIIIa. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8024–8028. doi: 10.1073/pnas.83.21.8024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Ichinose A., Hendrickson L. E., Fujikawa K., Davie E. W. Amino acid sequence of the a subunit of human factor XIII. Biochemistry. 1986 Nov 4;25(22):6900–6906. doi: 10.1021/bi00370a025. [DOI] [PubMed] [Google Scholar]
- Janus T. J., Lewis S. D., Lorand L., Shafer J. A. Promotion of thrombin-catalyzed activation of factor XIII by fibrinogen. Biochemistry. 1983 Dec 20;22(26):6269–6272. doi: 10.1021/bi00295a035. [DOI] [PubMed] [Google Scholar]
- LORAND L., DICKENMAN R. C. Assay method for the fibrin-stabilizing factor. Proc Soc Exp Biol Med. 1955 May;89(1):45–48. doi: 10.3181/00379727-89-21711. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewis S. D., Janus T. J., Lorand L., Shafer J. A. Regulation of formation of factor XIIIa by its fibrin substrates. Biochemistry. 1985 Nov 19;24(24):6772–6777. doi: 10.1021/bi00345a007. [DOI] [PubMed] [Google Scholar]
- Lorand L. Activation of blood coagulation factor XIII. Ann N Y Acad Sci. 1986;485:144–158. doi: 10.1111/j.1749-6632.1986.tb34577.x. [DOI] [PubMed] [Google Scholar]
- Mary A., Achyuthan K. E., Greenberg C. S. The binding of divalent metal ions to platelet factor XIII modulates its proteolysis by trypsin and thrombin. Arch Biochem Biophys. 1988 Feb 15;261(1):112–121. doi: 10.1016/0003-9861(88)90110-5. [DOI] [PubMed] [Google Scholar]
- Miraglia C. C., Greenberg C. S. Measurement of blood coagulation Factor XIIIa formation in plasma containing glycyl-L-prolyl-L-arginyl-L-proline. Anal Biochem. 1985 Jan;144(1):165–171. doi: 10.1016/0003-2697(85)90099-5. [DOI] [PubMed] [Google Scholar]
- Schwartz M. L., Pizzo S. V., Hill R. L., McKee P. A. The subunit structures of human plasma and platelet factor XIII (fibrin-stabilizing factor). J Biol Chem. 1971 Sep 25;246(18):5851–5854. [PubMed] [Google Scholar]
- Skrzynia C., Reisner H. M., McDonagh J. Characterization of the catalytic subunit of factor XIII by radioimmunoassay. Blood. 1982 Nov;60(5):1089–1095. [PubMed] [Google Scholar]
- Takagi T., Doolittle R. F. Amino acid sequence studies on factor XIII and the peptide released during its activation by thrombin. Biochemistry. 1974 Feb 12;13(4):750–756. doi: 10.1021/bi00701a018. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Takahashi Y., Putnam F. W. Primary structure of blood coagulation factor XIIIa (fibrinoligase, transglutaminase) from human placenta. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8019–8023. doi: 10.1073/pnas.83.21.8019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg L. J., Shiu D. T., Greenberg C. S., Kan Y. W., Shuman M. A. Localization of the gene for coagulation factor XIII a-chain to chromosome 6 and identification of sites of synthesis. J Clin Invest. 1987 Feb;79(2):649–652. doi: 10.1172/JCI112862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiman B., Wallén P. The specific interaction between plasminogen and fibrin. A physiological role of the lysine binding site in plasminogen. Thromb Res. 1977 Feb;10(2):213–222. doi: 10.1016/0049-3848(77)90003-2. [DOI] [PubMed] [Google Scholar]