Abstract
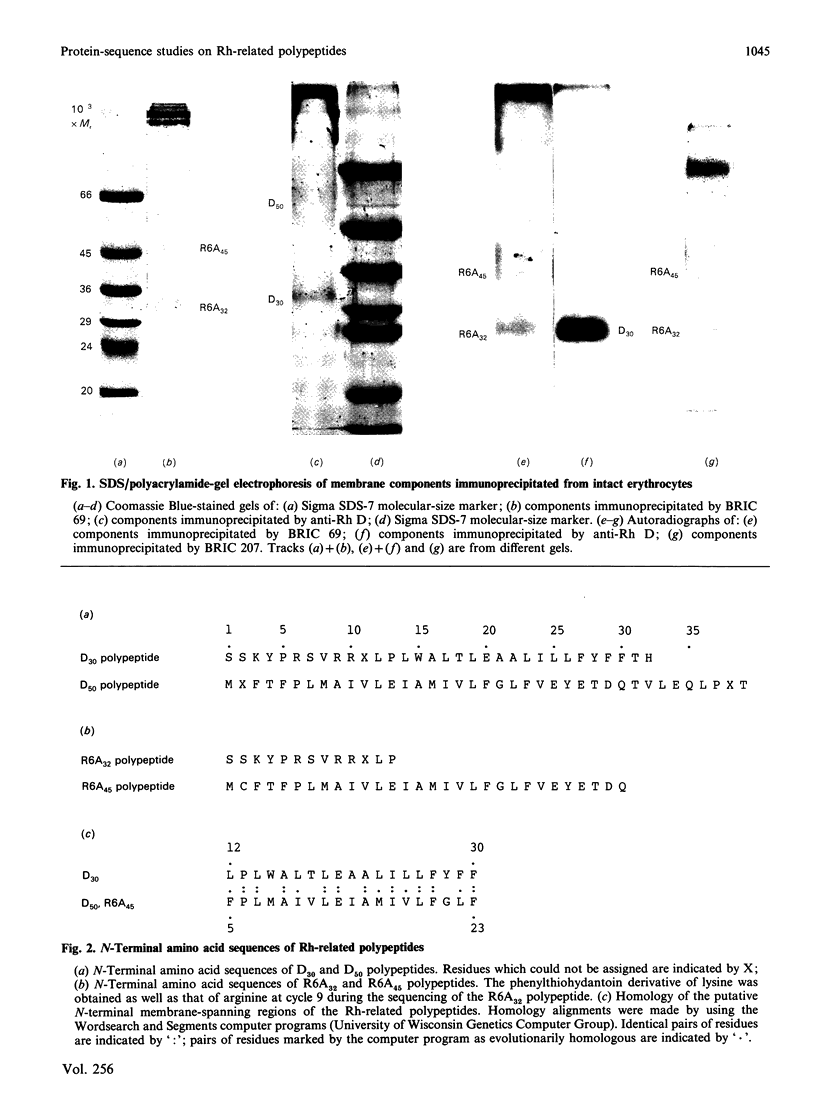
The Rh D blood-group antigen forms part of a complex, involving several other polypeptides, that is deficient in the red cells of individuals who lack all the antigens of the Rh blood-group system (Rhnull red cells). These include components recognized by anti-(Rh D) antibodies and the murine monoclonal antibodies R6A and BRIC 125. We have carried out protein-sequence studies on the components immunoprecipitated by these antibodies. Anti-(Rh D) antibodies immunoprecipitate an Mr-30,000-32,000 polypeptide (the D30 polypeptide) and an Mr-45,000-100,000 glycoprotein (D50 polypeptide). Antibody R6A immunoprecipitates two glycoproteins of Mr 31,000-34,000 (R6A32 polypeptide) and Mr 35,000-52,000 (R6A45 polypeptide). The D30 and R6A32 polypeptides were found to have the same N-terminal amino acid sequences, showing that they are closely related proteins. The D50 polypeptide and the R6A45 polypeptide also had indistinguishable N-terminal amino acid sequences that differed from that of the D30 and R6A32 polypeptides. The putative N-terminal membrane-spanning segments of the two groups of proteins showed homology in their amino acid sequence, which may account for the association of each of the pairs of proteins during co-precipitation by the antibodies. Supplementary data related to the protein sequence have been deposited as Supplementary Publication SUP 50417 (6 pages) at the British Library Document Supply Centre, Boston Spa, Wetherby, West Yorkshire LS23 7BQ, U.K., from whom copies can be obtained on the terms indicated in Biochem. J. (1988) 249, 5.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amons R. Vapor-phase modification of sulfhydryl groups in proteins. FEBS Lett. 1987 Feb 9;212(1):68–72. doi: 10.1016/0014-5793(87)81558-2. [DOI] [PubMed] [Google Scholar]
- Anstee D. J., Edwards P. A. Monoclonal antibodies to human erythrocytes. Eur J Immunol. 1982 Mar;12(3):228–232. doi: 10.1002/eji.1830120311. [DOI] [PubMed] [Google Scholar]
- Avent N., Judson P. A., Parsons S. F., Mallinson G., Anstee D. J., Tanner M. J., Evans P. R., Hodges E., Maciver A. G., Holmes C. Monoclonal antibodies that recognize different membrane proteins that are deficient in Rhnull human erythrocytes. One group of antibodies reacts with a variety of cells and tissues whereas the other group is erythroid-specific. Biochem J. 1988 Apr 15;251(2):499–505. doi: 10.1042/bj2510499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloy C., Blanchard D., Dahr W., Beyreuther K., Salmon C., Cartron J. P. Determination of the N-terminal sequence of human red cell Rh(D) polypeptide and demonstration that the Rh(D), (c), and (E) antigens are carried by distinct polypeptide chains. Blood. 1988 Aug;72(2):661–666. [PubMed] [Google Scholar]
- Dahr W., Kordowicz M., Moulds J., Gielen W., Lebeck L., Krüger J. Characterization of the Ss sialoglycoprotein and its antigens in Rhnull erythrocytes. Blut. 1987 Jan;54(1):13–24. doi: 10.1007/BF00326022. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G. Molecular identification of the human Rho (D) antigen. FEBS Lett. 1982 Apr 5;140(1):93–97. doi: 10.1016/0014-5793(82)80528-0. [DOI] [PubMed] [Google Scholar]
- Hughes-Jones N. C., Gardner B., Lincoln P. J. Observations of the number of available c,D, and E antigen sites on red cells. Vox Sang. 1971 Sep;21(3):210–216. doi: 10.1111/j.1423-0410.1971.tb00578.x. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mallinson G., Martin P. G., Anstee D. J., Tanner M. J., Merry A. H., Tills D., Sonneborn H. H. Identification and partial characterization of the human erythrocyte membrane component(s) that express the antigens of the LW blood-group system. Biochem J. 1986 Mar 15;234(3):649–652. doi: 10.1042/bj2340649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S., Green C. The identification of specific Rhesus-polypeptide-blood-group-ABH-active-glycoprotein complexes in the human red-cell membrane. Biochem J. 1987 Jun 15;244(3):735–741. doi: 10.1042/bj2440735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S., Woodrow C. F., McClelland D. B. Isolation of membrane components associated with human red cell antigens Rh(D), (c), (E) and Fy. Nature. 1982 Feb 11;295(5849):529–531. doi: 10.1038/295529a0. [DOI] [PubMed] [Google Scholar]
- Ridgwell K., Roberts S. J., Tanner M. J., Anstee D. J. Absence of two membrane proteins containing extracellular thiol groups in Rhnull human erythrocytes. Biochem J. 1983 Jul 1;213(1):267–269. doi: 10.1042/bj2130267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saboori A. M., Smith B. L., Agre P. Polymorphism in the Mr 32,000 Rh protein purified from Rh(D)-positive and -negative erythrocytes. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4042–4045. doi: 10.1073/pnas.85.11.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel D., Flügge U. I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984 Apr;138(1):141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]



