Abstract
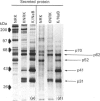
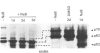
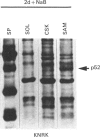
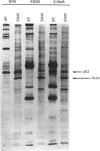
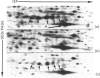
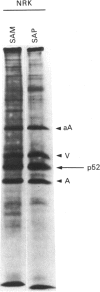
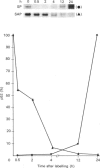
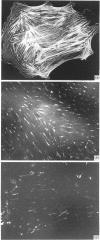
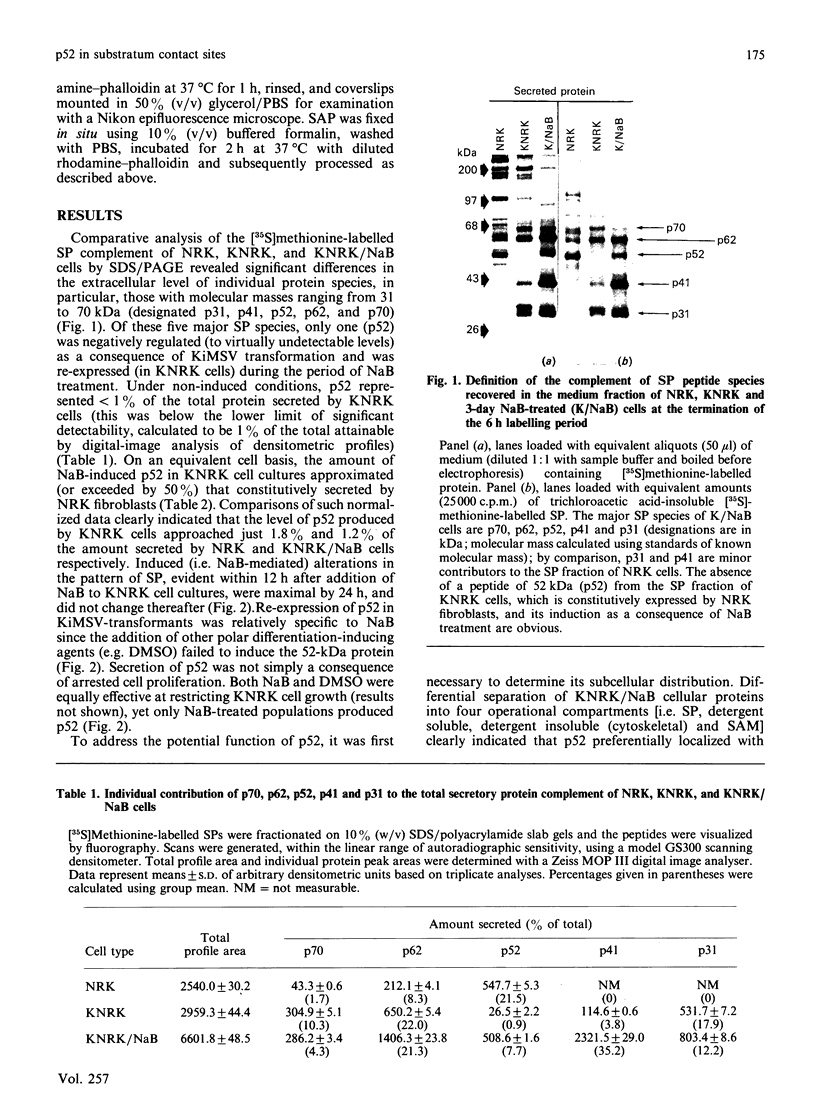
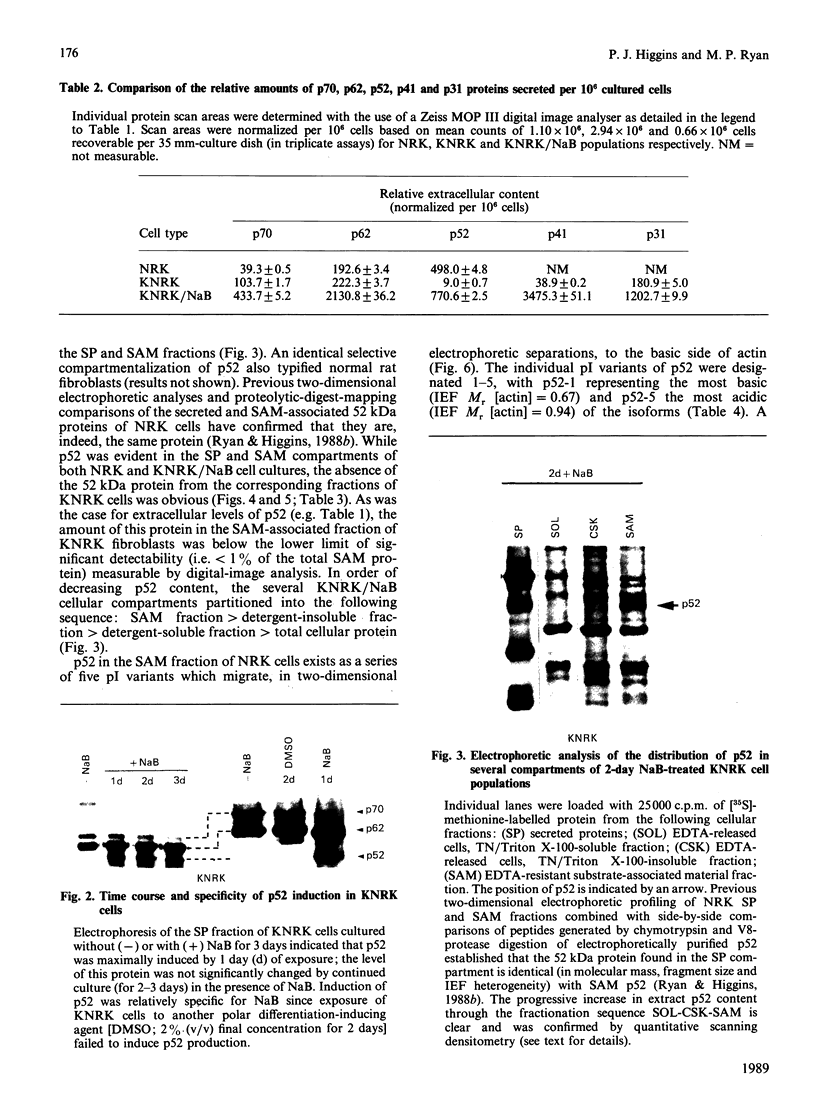
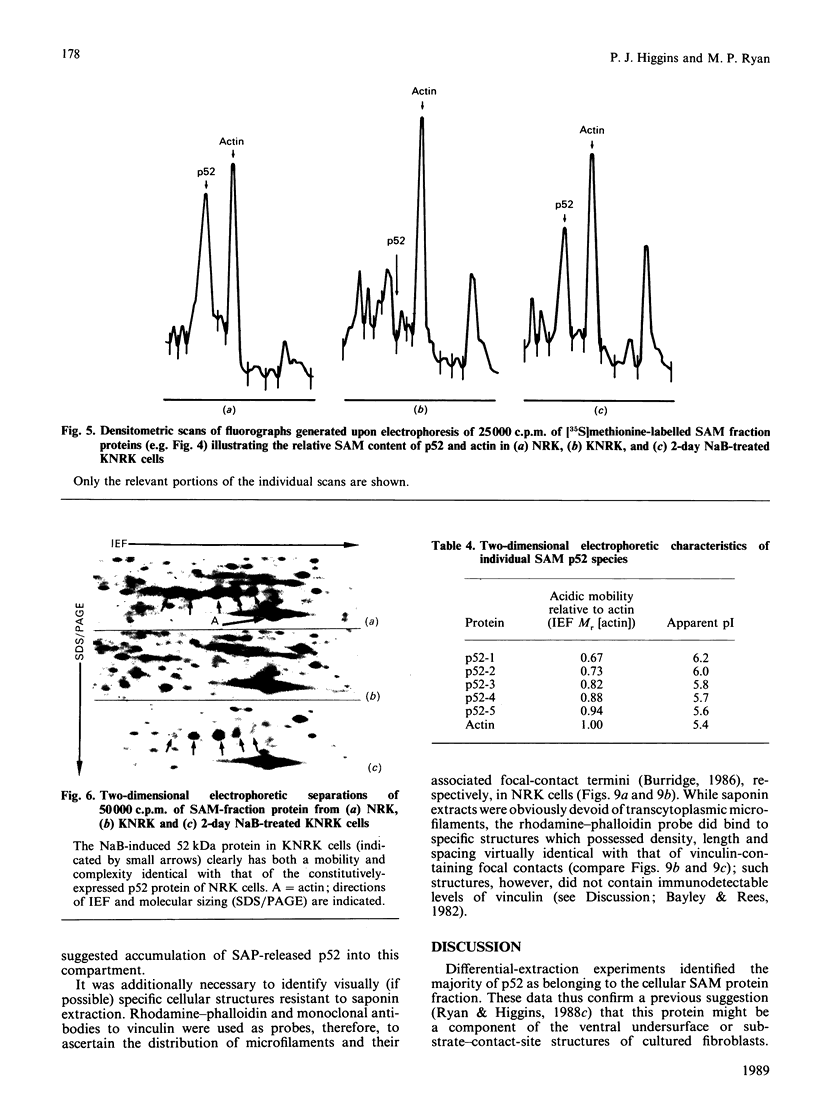
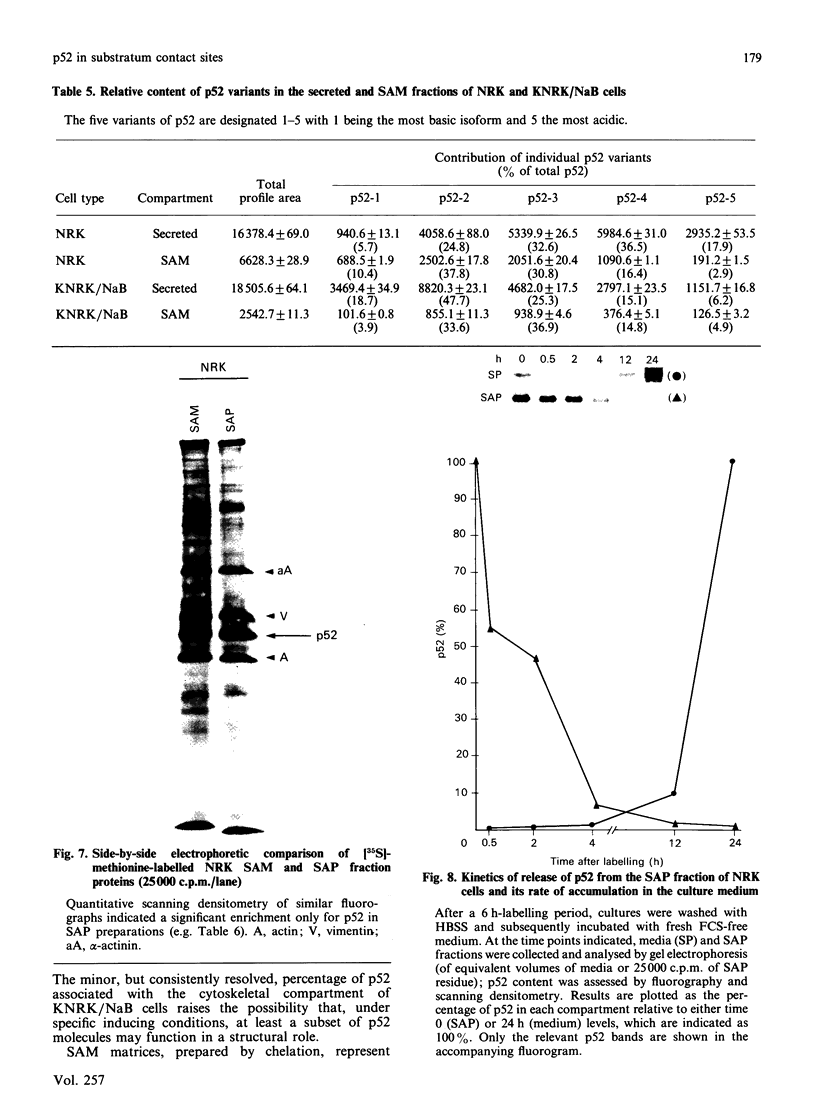
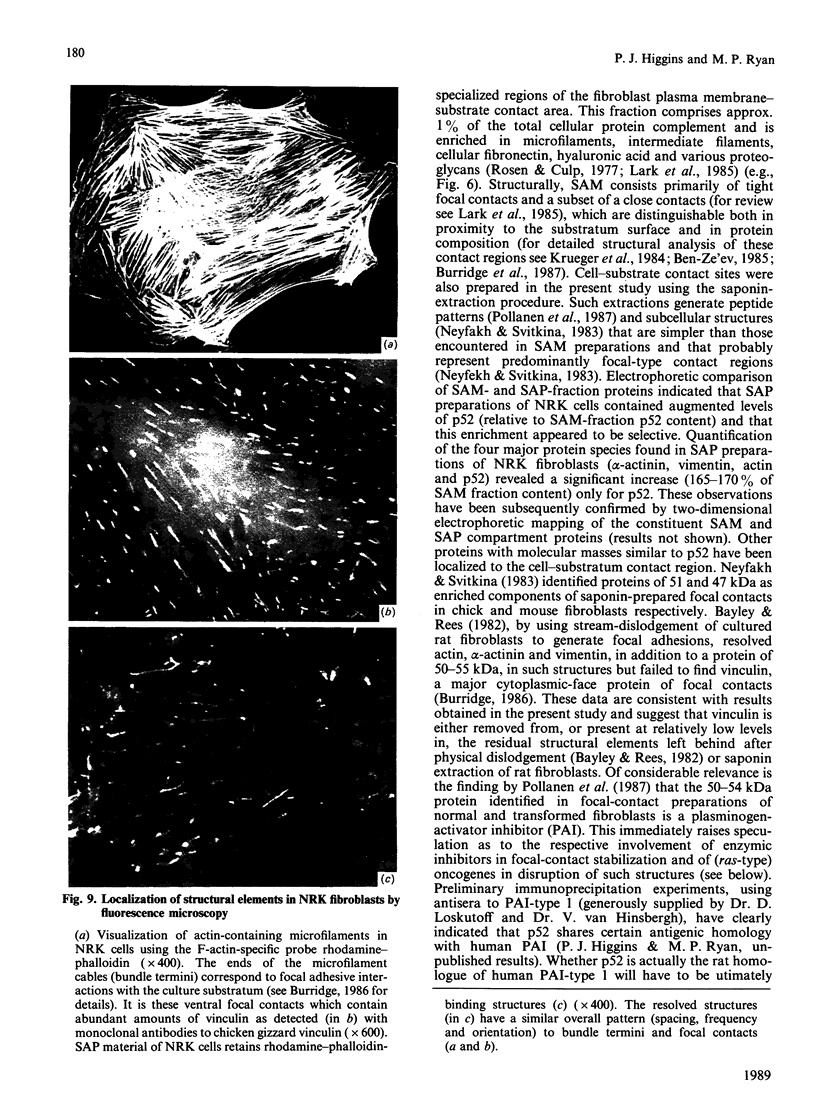
A 52 kDa protein (p52) was identified, using differential extraction and electrophoretic criteria, as a major extracellular and substrate-associated component of normal rat kidney (NRK) fibroblasts. Cells transformed with Kirsten murine sarcoma virus (KNRK cells) did not express p52 constitutively, but were inducible for both p52 production and its substrate association during culture in sodium butyrate (NaB)-supplemented growth medium. Comparative analysis of the relative molecular mass, subcellular distribution, and isoelectric complexity (five variants ranging in pI from 5.4 to 6.2) of the 52 kDa species constitutively and inducibly expressed by NRK and KNRK/NaB cells respectively, indicated that they were, indeed, the same protein. p52 selectively localized to cellular fractions enriched in substrate focal contact sites and associated ventral undersurface components. NaB induction of p52 in KNRK cells occurred before cell spreading; other polar compounds, such as dimethyl sulphoxide, which did not induce KNRK cell spreading, similarly failed to elicit p52 production. p52 accumulated more rapidly in (and was quickly released from) the focal-contact-enriched protein fraction of NRK cells compared with its time course of appearance in the medium. These data collectively suggest that p52 is one of a relatively small number of proteins the synthesis of which is either involved in determination of cell shape or regulated as a consequence of cell-shape changes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali I. U., Mautner V., Lanza R., Hynes R. O. Restoration of normal morphology, adhesion and cytoskeleton in transformed cells by addition of a transformation-sensitive surface protein. Cell. 1977 May;11(1):115–126. doi: 10.1016/0092-8674(77)90322-1. [DOI] [PubMed] [Google Scholar]
- Altenburg B. C., Via D. P., Steiner S. H. Modification of the phenotype of murine sarcoma virus-transformed cells by sodium butyrate. Effects on morphology and cytoskeletal elements. Exp Cell Res. 1976 Oct 15;102(2):223–231. doi: 10.1016/0014-4827(76)90036-7. [DOI] [PubMed] [Google Scholar]
- Bayley S. A., Rees D. A. Analysis of the proteins, glycoproteins and glycosaminoglycans of fibroblast adhesions to substratum. Biochim Biophys Acta. 1982 Jul 28;689(2):351–362. doi: 10.1016/0005-2736(82)90269-3. [DOI] [PubMed] [Google Scholar]
- Ben-Ze'ev A. The cytoskeleton in cancer cells. Biochim Biophys Acta. 1985;780(3):197–212. doi: 10.1016/0304-419x(85)90003-4. [DOI] [PubMed] [Google Scholar]
- Burridge K., Molony L., Kelly T. Adhesion plaques: sites of transmembrane interaction between the extracellular matrix and the actin cytoskeleton. J Cell Sci Suppl. 1987;8:211–229. doi: 10.1242/jcs.1987.supplement_8.12. [DOI] [PubMed] [Google Scholar]
- Carley W. W., Barak L. S., Webb W. W. F-actin aggregates in transformed cells. J Cell Biol. 1981 Sep;90(3):797–802. doi: 10.1083/jcb.90.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper H. L., Feuerstein N., Noda M., Bassin R. H. Suppression of tropomyosin synthesis, a common biochemical feature of oncogenesis by structurally diverse retroviral oncogenes. Mol Cell Biol. 1985 May;5(5):972–983. doi: 10.1128/mcb.5.5.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan S. E., Wright J. A., Jarolim L., Yanagihara K., Bassin R. H., Greenberg A. H. Transformation by oncogenes encoding protein kinases induces the metastatic phenotype. Science. 1987 Oct 9;238(4824):202–205. doi: 10.1126/science.3659911. [DOI] [PubMed] [Google Scholar]
- Erlandson R. A., Cardon-Cardo C., Higgins P. J. Histogenesis of benign pleomorphic adenoma (mixed tumor) of the major salivary glands. An ultrastructural and immunohistochemical study. Am J Surg Pathol. 1984 Nov;8(11):803–820. doi: 10.1097/00000478-198411000-00001. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Denk H., Kalt R., Schmid E. Biochemical and immunological identification of cytokeratin proteins present in hepatocytes of mammalian liver tissue. Exp Cell Res. 1981 Feb;131(2):299–318. doi: 10.1016/0014-4827(81)90234-2. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Mayer D., Schmid E., Denk H., Borenfreund E. Differences of expression of cytoskeletal proteins in cultured rat hepatocytes and hepatoma cells. Exp Cell Res. 1981 Aug;134(2):345–365. doi: 10.1016/0014-4827(81)90435-3. [DOI] [PubMed] [Google Scholar]
- Hayman E. G., Engvall E., Ruoslahti E. Concomitant loss of cell surface fibronectin and laminin from transformed rat kidney cells. J Cell Biol. 1981 Feb;88(2):352–357. doi: 10.1083/jcb.88.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins P. J. Characterization of the growth inhibited substate induced in murine hepatic tumor cells during in vitro exposure to dimethylsulfoxide. Int J Cancer. 1986 Dec 15;38(6):889–899. doi: 10.1002/ijc.2910380617. [DOI] [PubMed] [Google Scholar]
- Higgins P. J., Lipkin G., Rosenberg M., Ryan M. P. Contact-inhibitory factor induces alterations in the distribution and content of specific cytoskeletal elements in an established line of rat hepatic tumor cells. Int J Cancer. 1987 Dec 15;40(6):792–801. doi: 10.1002/ijc.2910400615. [DOI] [PubMed] [Google Scholar]
- Krueger J. G., Garber E. A., Chin S. S., Hanafusa H., Goldberg A. R. Size-variant pp60src proteins of recovered avian sarcoma viruses interact with adhesion plaques as peripheral membrane proteins: effects on cell transformation. Mol Cell Biol. 1984 Mar;4(3):454–467. doi: 10.1128/mcb.4.3.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lark M. W., Laterra J., Culp L. A. Close and focal contact adhesions of fibroblasts to a fibronectin-containing matrix. Fed Proc. 1985 Feb;44(2):394–403. [PubMed] [Google Scholar]
- Matsumura F., Lin J. J., Yamashiro-Matsumura S., Thomas G. P., Topp W. C. Differential expression of tropomyosin forms in the microfilaments isolated from normal and transformed rat cultured cells. J Biol Chem. 1983 Nov 25;258(22):13954–13964. [PubMed] [Google Scholar]
- Neyfakh A. A., Jr, Svitkina T. M. Isolation of focal contact membrane using saponin. Exp Cell Res. 1983 Dec;149(2):582–586. doi: 10.1016/0014-4827(83)90369-5. [DOI] [PubMed] [Google Scholar]
- Pöllänen J., Saksela O., Salonen E. M., Andreasen P., Nielsen L., Danø K., Vaheri A. Distinct localizations of urokinase-type plasminogen activator and its type 1 inhibitor under cultured human fibroblasts and sarcoma cells. J Cell Biol. 1987 Apr;104(4):1085–1096. doi: 10.1083/jcb.104.4.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheinwald J. G., Jorgensen J. L., Hahn W. C., Terpstra A. J., O'Connell T. M., Plummer K. K. Mesosecrin: a secreted glycoprotein produced in abundance by human mesothelial, endothelial, and kidney epithelial cells in culture. J Cell Biol. 1987 Feb;104(2):263–275. doi: 10.1083/jcb.104.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen J. J., Culp L. A. Morphology and cellular origins of substrate-attached material from mouse fibroblasts. Exp Cell Res. 1977 Jun;107(1):139–149. doi: 10.1016/0014-4827(77)90395-0. [DOI] [PubMed] [Google Scholar]
- Ryan M. P., Borenfreund E., Higgins P. J. Butyrate-induced cytoarchitectural reorganization of Mallory body-containing rat hepatic tumor cells. J Natl Cancer Inst. 1987 Sep;79(3):555–567. [PubMed] [Google Scholar]
- Ryan M. P., Higgins P. J. Discrimination between the nuclear lamin and intermediate filament (cytokeratin/vimentin) proteins of rat hepatic tumor cells by differential solubility and electrophoretic criteria. Int J Biochem. 1987;19(12):1187–1192. doi: 10.1016/0020-711x(87)90101-7. [DOI] [PubMed] [Google Scholar]
- Stickel S. K., Wang Y. L. Alpha-actinin-containing aggregates in transformed cells are highly dynamic structures. J Cell Biol. 1987 Jun;104(6):1521–1526. doi: 10.1083/jcb.104.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verderame M., Alcorta D., Egnor M., Smith K., Pollack R. Cytoskeletal F-actin patterns quantitated with fluorescein isothiocyanate-phalloidin in normal and transformed cells. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6624–6628. doi: 10.1073/pnas.77.11.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Via D. P., Sramek S., Larriba G., Steiner S. Effects of sodium butyrate on the membrane glycoconjugates of murine sarcoma virus-transformed rat cells. J Cell Biol. 1980 Feb;84(2):225–234. doi: 10.1083/jcb.84.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahrman M. Z., Gagnier S. E., Kobrin D. R., Higgins P. J., Augenlicht L. H. Cellular and molecular changes in 3T3 cells transformed spontaneously or by DNA transfection. Tumour Biol. 1985;6(1):41–56. [PubMed] [Google Scholar]
- Wang E., Goldberg A. R. Changes in microfilament organization and surface topogrophy upon transformation of chick embryo fibroblasts with Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4065–4069. doi: 10.1073/pnas.73.11.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulf E., Deboben A., Bautz F. A., Faulstich H., Wieland T. Fluorescent phallotoxin, a tool for the visualization of cellular actin. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4498–4502. doi: 10.1073/pnas.76.9.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]