Abstract
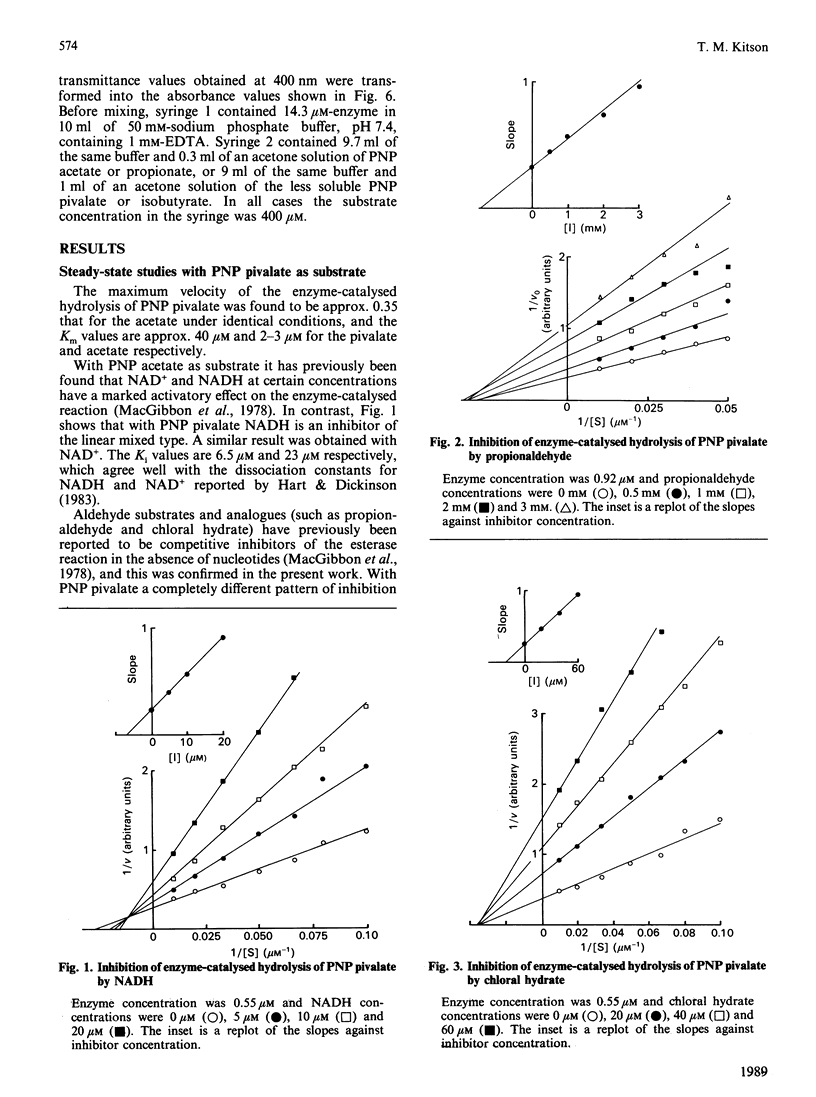
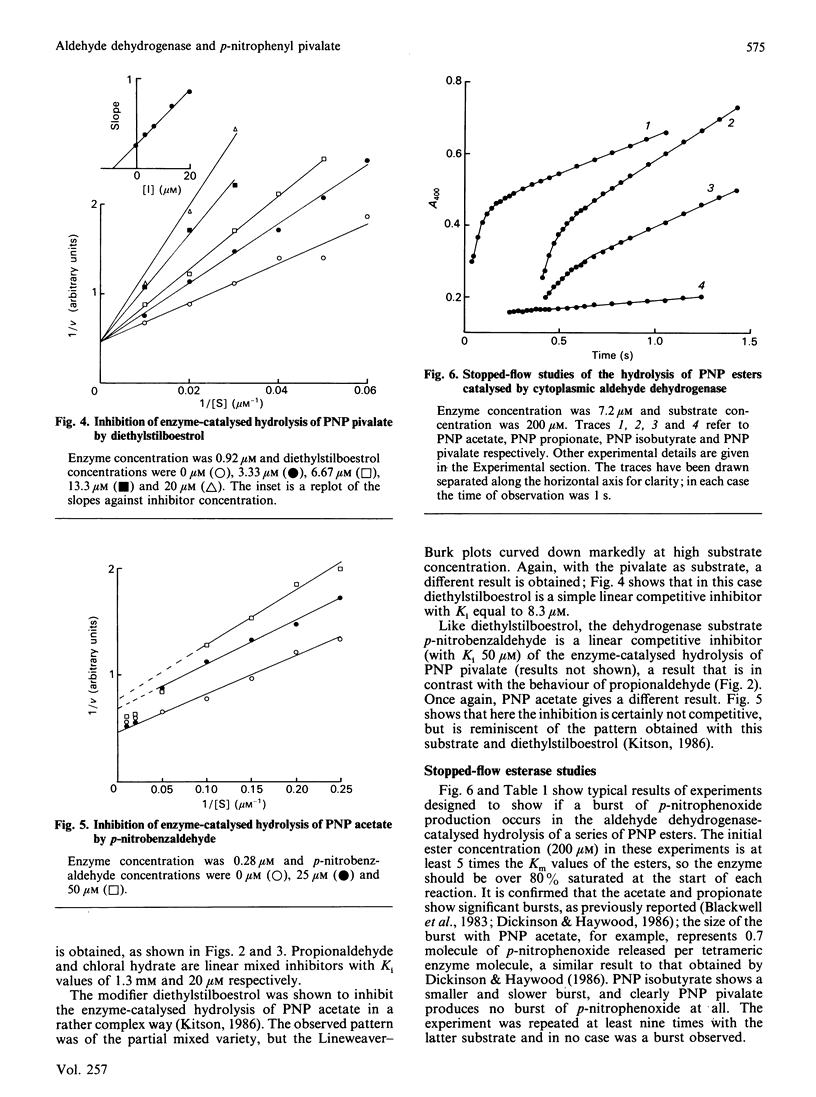
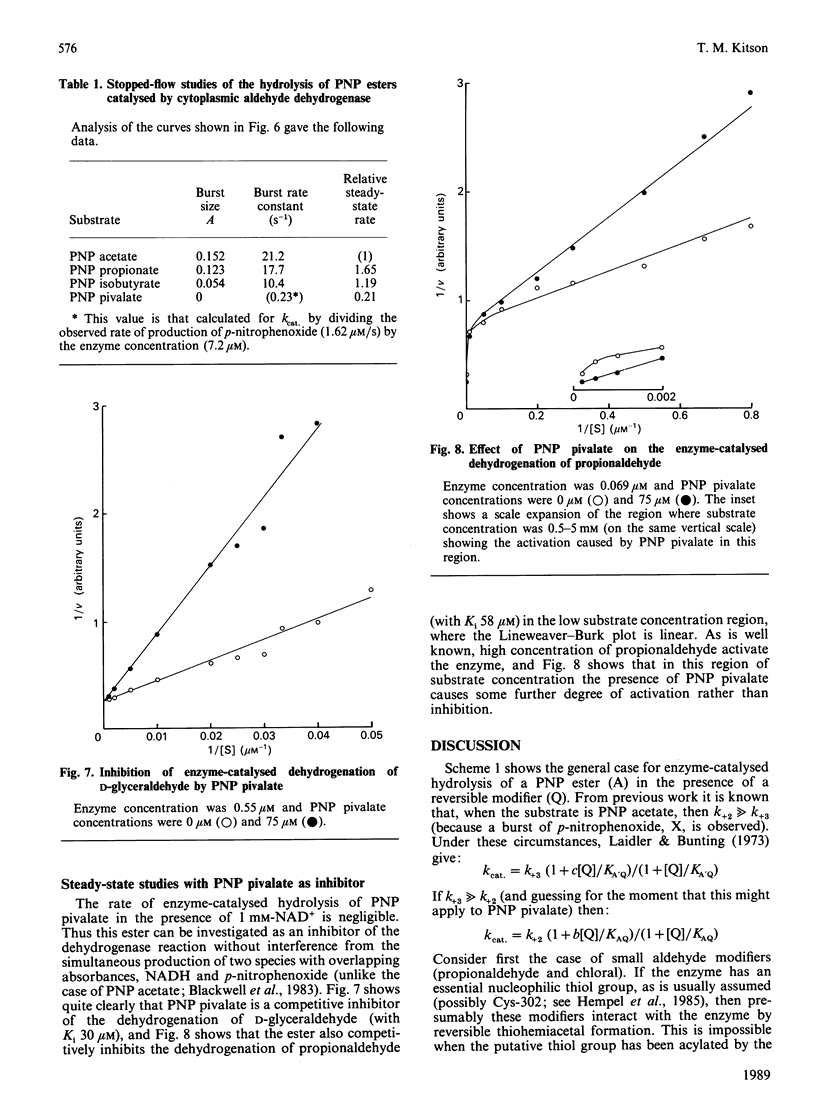
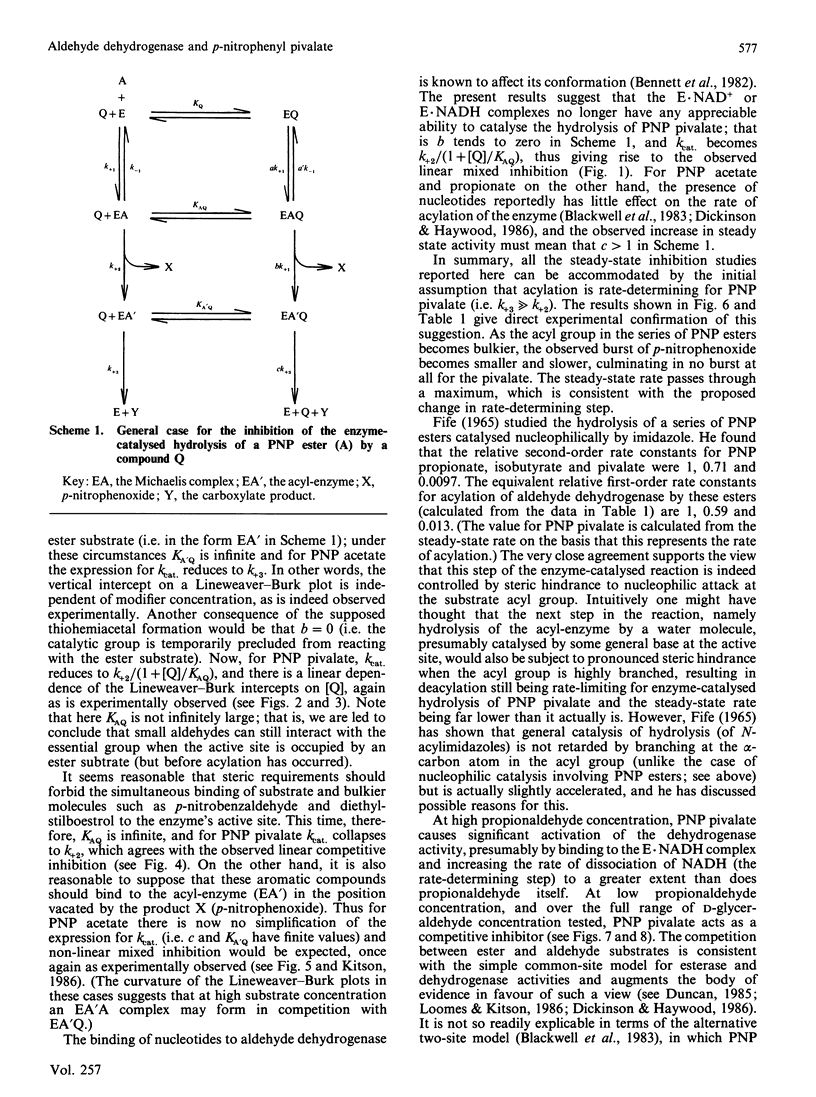
The effects of modifiers (NAD+, NADH, propionaldehyde, chloral hydrate, diethylstilboestrol and p-nitrobenzaldehyde) on the hydrolysis of p-nitrophenyl (PNP) pivalate (PNP trimethylacetate) catalysed by cytoplasmic aldehyde dehydrogenase are reported. In each case a different inhibition pattern is obtained to that observed when the substrate is PNP acetate; for example, propionaldehyde and chloral hydrate competitively inhibit the hydrolysis of PNP acetate, but are mixed inhibitors with PNP pivalate. The kinetic results can be rationalized in terms of different rate-determining steps: acylation of the enzyme in the case of the pivalate but acyl-enzyme hydrolysis for the acetate. This is confirmed by stopped-flow studies, in which a burst of p-nitrophenoxide is observed when the substrate is PNP acetate, but not when it is the pivalate. PNP pivalate inhibits the dehydrogenase activity of the enzyme competitively with the aldehyde substrate; this is most simply explained if the esterase and dehydrogenase reactions occur at a common enzymic site.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bender M. L., Kézdy F. J., Wedler F. C. Alpha-chymotrypsin: enzyme concentration and kinetics. J Chem Educ. 1967 Feb;44(2):84–88. doi: 10.1021/ed044p84. [DOI] [PubMed] [Google Scholar]
- Bennett A. F., Buckley P. D., Blackwell L. F. Proton release during the pre-steady-state oxidation of aldehydes by aldehyde dehydrogenase. Evidence for a rate-limiting conformational change. Biochemistry. 1982 Aug 31;21(18):4407–4413. doi: 10.1021/bi00261a033. [DOI] [PubMed] [Google Scholar]
- Blackwell L. F., Bennett A. F., Buckley P. D. Relationship between the mechanisms of the esterase and dehydrogenase activities of the cytoplasmic aldehyde dehydrogenase from sheep liver. An alternative view. Biochemistry. 1983 Aug 2;22(16):3784–3791. doi: 10.1021/bi00285a011. [DOI] [PubMed] [Google Scholar]
- Deady L. W., Buckley P. D., Bennett A. F., Blackwell L. F. Kinetics of inhibition and hysteresis of sheep liver cytoplasmic aldehyde dehydrogenase with glyoxylic acid: further evidence relating to the two-site model for aldehyde oxidation. Arch Biochem Biophys. 1985 Dec;243(2):586–597. doi: 10.1016/0003-9861(85)90536-3. [DOI] [PubMed] [Google Scholar]
- Dickinson F. M., Hart G. J. Effects of Mg2+, Ca2+ and Mn2+ on sheep liver cytoplasmic aldehyde dehydrogenase. Biochem J. 1982 Aug 1;205(2):443–448. doi: 10.1042/bj2050443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson F. M., Hart G. J., Kitson T. M. The use of pH-gradient ion-exchange chromatography to separate sheep liver cytoplasmic aldehyde dehydrogenase from mitochondrial enzyme contamination, and observations on the interaction between the pure cytoplasmic enzyme and disulfiram. Biochem J. 1981 Dec 1;199(3):573–579. doi: 10.1042/bj1990573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson F. M., Haywood G. W. The effects of Mg2+ on certain steps in the mechanisms of the dehydrogenase and esterase reactions catalysed by sheep liver aldehyde dehydrogenase. Support for the view that dehydrogenase and esterase activities occur at the same site on the enzyme. Biochem J. 1986 Feb 1;233(3):877–883. doi: 10.1042/bj2330877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan R. J. Aldehyde dehydrogenase. An enzyme with two distinct catalytic activities at a single type of active site. Biochem J. 1985 Aug 15;230(1):261–267. doi: 10.1042/bj2300261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R. I., Weiner H. Horse liver aldehyde dehydrogenase. II. Kinetics and mechanistic implications of the dehydrogenase and esterase activity. J Biol Chem. 1972 Jan 10;247(1):267–272. [PubMed] [Google Scholar]
- Hart G. J., Dickinson F. M. Kinetic properties of highly purified preparations of sheep liver cytoplasmic aldehyde dehydrogenase. Biochem J. 1982 Jun 1;203(3):617–627. doi: 10.1042/bj2030617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart G. J., Dickinson F. M. Some properties of aldehyde dehydrogenase from sheep liver mitochondria. Biochem J. 1977 May 1;163(2):261–267. doi: 10.1042/bj1630261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart G. J., Dickinson F. M. The coenzyme-binding characteristics of highly purified preparations of sheep liver cytoplasmic aldehyde dehydrogenase. Biochem J. 1983 May 1;211(2):363–371. doi: 10.1042/bj2110363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel J., Kaiser R., Jörnvall H. Mitochondrial aldehyde dehydrogenase from human liver. Primary structure, differences in relation to the cytosolic enzyme, and functional correlations. Eur J Biochem. 1985 Nov 15;153(1):13–28. doi: 10.1111/j.1432-1033.1985.tb09260.x. [DOI] [PubMed] [Google Scholar]
- Kitson T. M. Effects of diethylstilbestrol, 2,2'-dithiodipyridine, and chloral hydrate on the esterase activity of sheep liver cytoplasmic aldehyde dehydrogenase. Biochemistry. 1986 Aug 12;25(16):4718–4724. doi: 10.1021/bi00364a040. [DOI] [PubMed] [Google Scholar]
- Loomes K. M., Kitson T. M. Aldehyde dehydrogenase catalyses acetaldehyde formation from 4-nitrophenyl acetate and NADH. Biochem J. 1986 Sep 1;238(2):617–619. doi: 10.1042/bj2380617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGibbon A. K., Haylock S. J., Buckley P. D., Blackwell L. F. Kinetic studies on the esterase activity of cytoplasmic sheep liver aldehyde dehydrogenase. Biochem J. 1978 Jun 1;171(3):533–538. doi: 10.1042/bj1710533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstien J. B., Fife T. H. Steric effects in the acylation of alpha-chymotrypsin. Biochemistry. 1969 Feb;8(2):623–627. doi: 10.1021/bi00830a024. [DOI] [PubMed] [Google Scholar]
- Sidhu R. S., Blair A. H. Human liver aldehyde dehydrogenase. Esterase activity. J Biol Chem. 1975 Oct 10;250(19):7894–7898. [PubMed] [Google Scholar]
- Takahashi K., Weiner H. Nicotinamide adenine dinucleotide activation of the esterase reaction of horse liver aldehyde dehydrogenase. Biochemistry. 1981 May 12;20(10):2720–2726. doi: 10.1021/bi00513a003. [DOI] [PubMed] [Google Scholar]


