Abstract
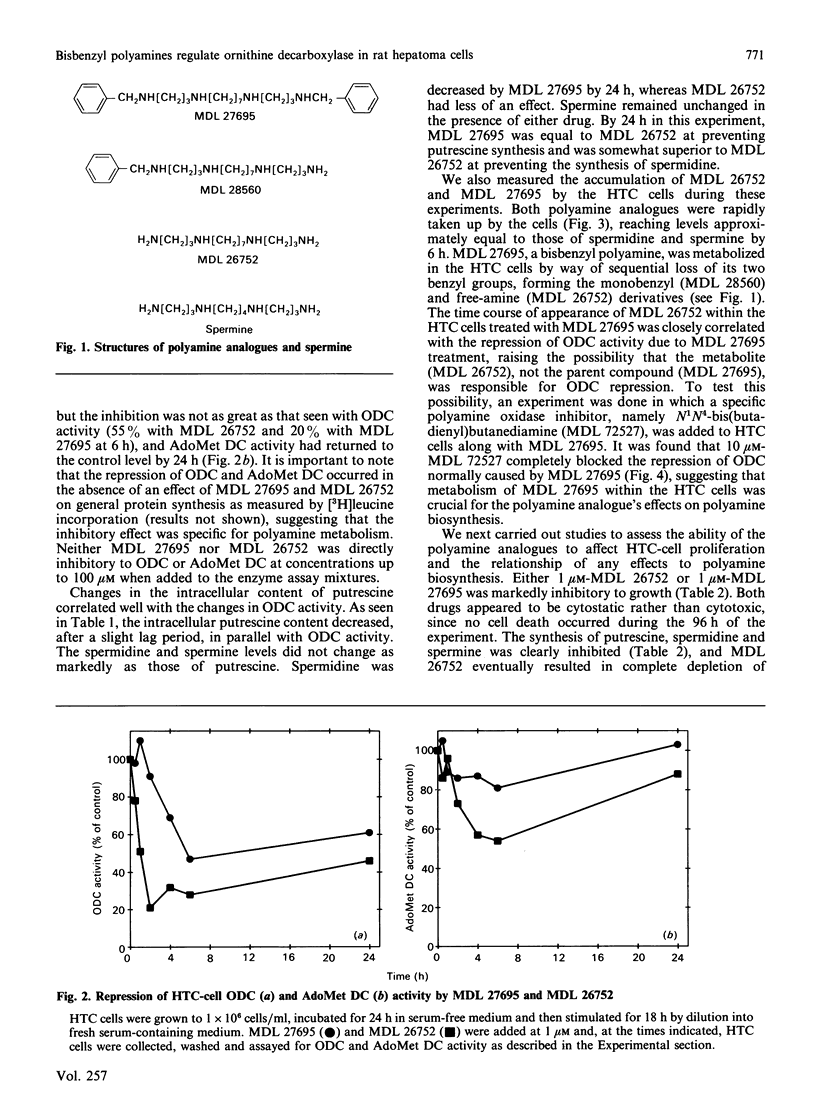
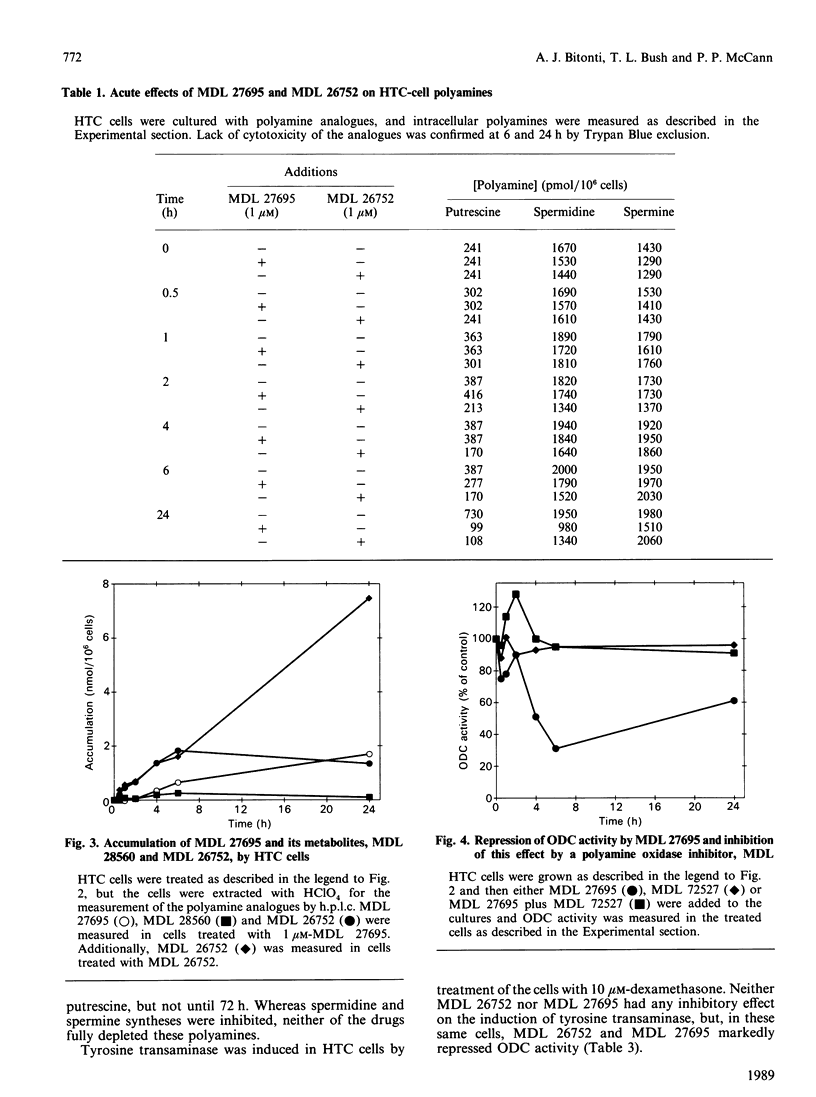
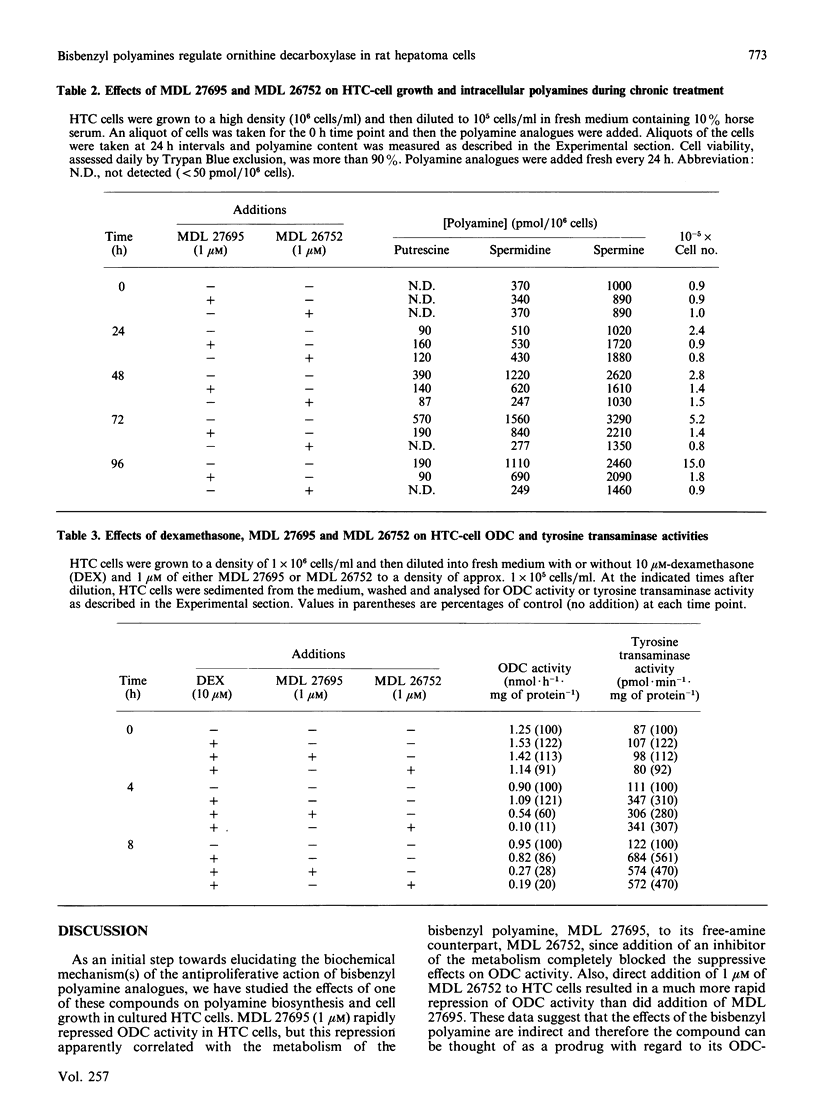
A bisbenzyl polyamine analogue, MDL 27695, rapidly repressed ornithine decarboxylase (ODC) and S-adenosyl-L-methionine decarboxylase (AdoMet DC) activity and depleted polyamines in rat hepatoma (HTC) cells. The suppression of ODC and AdoMet DC activity was temporally related to metabolism of MDL 27695 by intracellular polyamine oxidase to a free-amine analogue, MDL 26752, which, when added directly to HTC cells, suppressed ODC activity and polyamine biosynthesis more rapidly and to a greater extent than did the bisbenzyl analogue. The ODC suppression caused by MDL 27695 was completely blocked by the addition of a polyamine oxidase inhibitor to the HTC-cell cultures along with MDL 27695. These data suggested that MDL 27695 acted as a prodrug, with metabolism to an active analogue being necessary for ODC repression to occur. MDL 27695 and MDL 26752 completely abolished division of HTC cells when added to cultures at 1 microM. This established them as being among the most potent antiproliferative polyamine analogues yet described. MDL 27695 has also been shown to possess significant antimalarial effects both in vitro and in vivo, and it is possible that the marked suppression of polyamine biosynthesis described herein may contribute to its anti-malarial effects as well as its antiproliferative effects in mammalian cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bey P., Bolkenius F. N., Seiler N., Casara P. N-2,3-Butadienyl-1,4-butanediamine derivatives: potent irreversible inactivators of mammalian polyamine oxidase. J Med Chem. 1985 Jan;28(1):1–2. doi: 10.1021/jm00379a001. [DOI] [PubMed] [Google Scholar]
- Bitonti A. J., Bacchi C. J., McCann P. P., Sjoerdsma A. Catalytic irreversible inhibition of Trypanosoma brucei brucei ornithine decarboxylase by substrate and product analogs and their effects on murine trypanosomiasis. Biochem Pharmacol. 1985 May 15;34(10):1773–1777. doi: 10.1016/0006-2952(85)90648-3. [DOI] [PubMed] [Google Scholar]
- Bitonti A. J., Bacchi C. J., McCann P. P., Sjoerdsma A. Uptake of alpha-difluoromethylornithine by Trypanosoma brucei brucei. Biochem Pharmacol. 1986 Jan 15;35(2):351–354. doi: 10.1016/0006-2952(86)90539-3. [DOI] [PubMed] [Google Scholar]
- Bitonti A. J., Dumont J. A., McCann P. P. Characterization of Trypanosoma brucei brucei S-adenosyl-L-methionine decarboxylase and its inhibition by Berenil, pentamidine and methylglyoxal bis(guanylhydrazone). Biochem J. 1986 Aug 1;237(3):685–689. doi: 10.1042/bj2370685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N. D., Strickler M. P., Whaun J. M. Femtomolar ion-pair high-performance liquid chromatographic method for determining Dns-polyamine derivatives of red blood cell extracts utilizing an automated polyamine analyzer. J Chromatogr. 1982 Aug 6;245(1):101–108. doi: 10.1016/s0021-9673(00)82479-6. [DOI] [PubMed] [Google Scholar]
- Clark J. L., Fuller J. L. Regulation of ornithine decarboxylase in 3T3 cells by putrescine and spermidine: indirect evidence for translational control. Biochemistry. 1975 Oct 7;14(20):4403–4409. doi: 10.1021/bi00691a010. [DOI] [PubMed] [Google Scholar]
- Hershko A., Tomkins G. M. Studies on the degradation of tyrosine aminotransferase in hepatoma cells in culture. Influence of the composition of the medium and adenosine triphosphate dependence. J Biol Chem. 1971 Feb 10;246(3):710–714. [PubMed] [Google Scholar]
- Kay J. E., Lindsay V. J. Control of ornithine decarboxylase activity in stimulated human lymphocytes by putrescine and spermidine. Biochem J. 1973 Apr;132(4):791–796. doi: 10.1042/bj1320791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN E. C., KNOX W. E. Adaptation of the rat liver tyrosine-alpha-ketoglutarate transaminase. Biochim Biophys Acta. 1957 Oct;26(1):85–88. doi: 10.1016/0006-3002(57)90057-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McCann P. P., Tardif C., Hornsperger J. M., Böhlen P. Two distinct mechanisms for ornithine decarboxylase regulation by polyamines in rat hepatoma cells. J Cell Physiol. 1979 May;99(2):183–190. doi: 10.1002/jcp.1040990204. [DOI] [PubMed] [Google Scholar]
- McCann P. P., Tardif C., Pegg A. E., Diekema K. The dual action of the non-physiological diamines 1,3 diaminopropane and cadaverine on ornithine decarboxylase of HTC cells. Life Sci. 1980 Jun 9;26(23):2003–2010. doi: 10.1016/0024-3205(80)90633-5. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., McCann P. P. Polyamine metabolism and function. Am J Physiol. 1982 Nov;243(5):C212–C221. doi: 10.1152/ajpcell.1982.243.5.C212. [DOI] [PubMed] [Google Scholar]
- Porter C. W., Berger F. G., Pegg A. E., Ganis B., Bergeron R. J. Regulation of ornithine decarboxylase activity by spermidine and the spermidine analogue N1N8-bis(ethyl)spermidine. Biochem J. 1987 Mar 1;242(2):433–440. doi: 10.1042/bj2420433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter C. W., McManis J., Casero R. A., Bergeron R. J. Relative abilities of bis(ethyl) derivatives of putrescine, spermidine, and spermine to regulate polyamine biosynthesis and inhibit L1210 leukemia cell growth. Cancer Res. 1987 Jun 1;47(11):2821–2825. [PubMed] [Google Scholar]
- Seiler N., Knödgen B. High-performance liquid chromatographic procedure for the simultaneous determination of the natural polyamines and their monoacetyl derivatives. J Chromatogr. 1980 Dec 12;221(2):227–235. doi: 10.1016/s0378-4347(00)84307-8. [DOI] [PubMed] [Google Scholar]
- Tomkins G. M., Thompson E. B., Hayashi S., Gelehrter T., Granner D., Peterkofsky B. Tyrosine transaminase induction in mammalian cells in tissue culture. Cold Spring Harb Symp Quant Biol. 1966;31:349–360. doi: 10.1101/sqb.1966.031.01.045. [DOI] [PubMed] [Google Scholar]


