Abstract
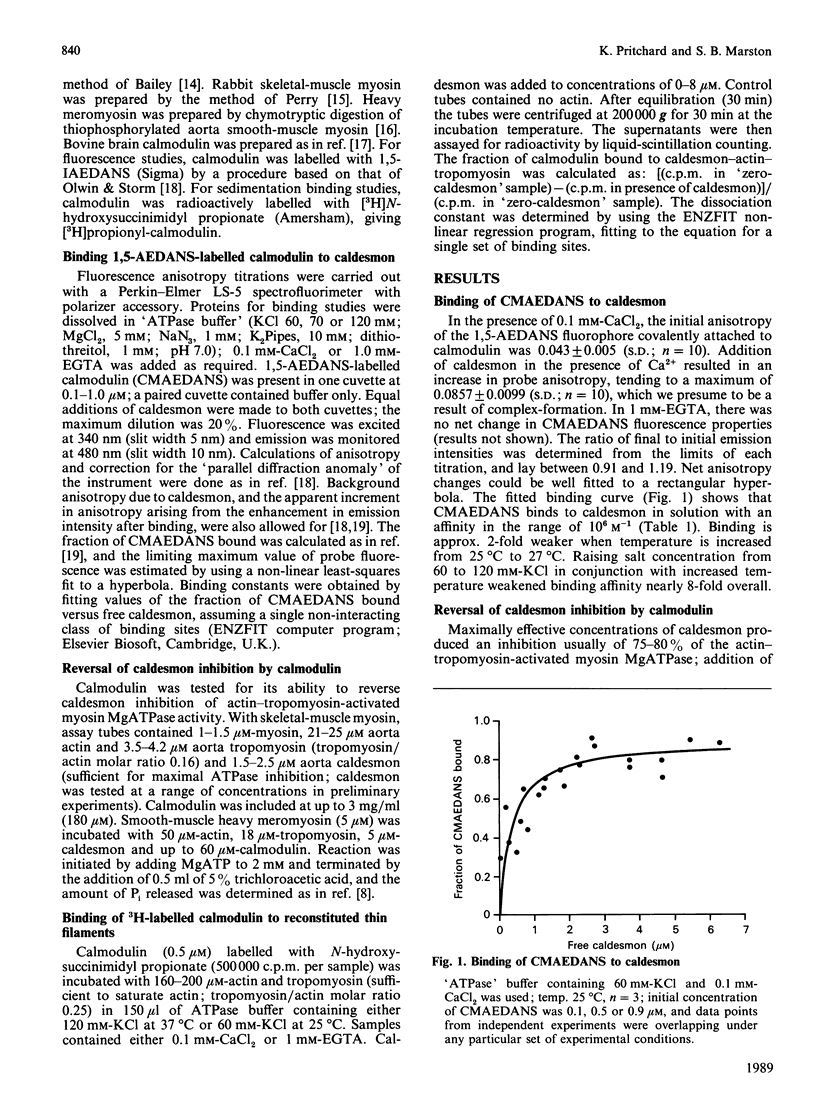
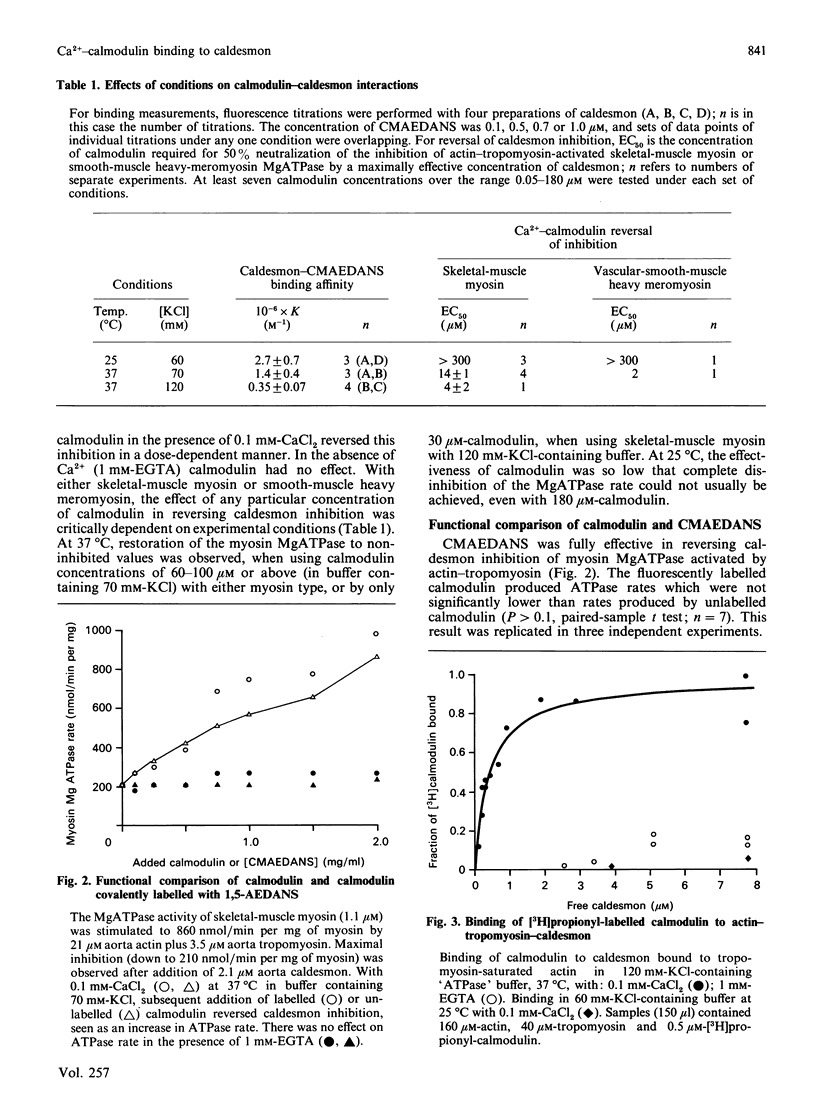
We measured the concentration of calmodulin required to reverse inhibition by caldesmon of actin-activated myosin MgATPase activity, in a model smooth-muscle thin-filament system, reconstituted in vitro from purified vascular smooth-muscle actin, tropomyosin and caldesmon. At 37 degrees C in buffer containing 120 mM-KCl, 4 microM-Ca2+-calmodulin produced a half-maximal reversal of caldesmon inhibition, but more than 300 microM-Ca2+-calmodulin was necessary at 25 degrees C in buffer containing 60 mM-KCl. The binding affinity (K) of caldesmon for Ca2+-calmodulin was measured by a fluorescence-polarization method: K = 2.7 x 10(6) M-1 at 25 degrees C (60 mM-KCl); K = 1.4 x 10(6) M-1 at 37 degrees C in 70 mM-KCl-containing buffer; K = 0.35 x 10(6) M-1 at 37 degrees C in 120 mM-KCl- containing buffer (pH 7.0). At 37 degrees C/120 mM-KCl, but not at 25 degrees C/60 mM-KCl, Ca2+-calmodulin bound to caldesmon bound to actin-tropomyosin (K = 2.9 x 10(6) M-1). Ca2+ regulation in this system does not depend on a simple competition between Ca2+-calmodulin and actin for binding to caldesmon. Under conditions (37 degrees C/120 mM-KCl) where physiologically realistic concentrations of calmodulin can Ca2+-regulate synthetic thin filaments, Ca2+-calmodulin reverses caldesmon inhibition of actomyosin ATPase by forming a non-inhibited complex of Ca2+-calmodulin-caldesmon-(actin-tropomyosin).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey K. Tropomyosin: a new asymmetric protein component of the muscle fibril. Biochem J. 1948;43(2):271–279. doi: 10.1042/bj0430271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A., Lynch W. Identification and localization of immunoreactive forms of caldesmon in smooth and nonmuscle cells: a comparison with the distributions of tropomyosin and alpha-actinin. J Cell Biol. 1985 May;100(5):1656–1663. doi: 10.1083/jcb.100.5.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschmeier B., Meyer H. E., Mayr G. W. Characterization of the calmodulin-binding sites of muscle phosphofructokinase and comparison with known calmodulin-binding domains. J Biol Chem. 1987 Jul 15;262(20):9454–9462. [PubMed] [Google Scholar]
- Clark T., Ngai P. K., Sutherland C., Gröschel-Stewart U., Walsh M. P. Vascular smooth muscle caldesmon. J Biol Chem. 1986 Jun 15;261(17):8028–8035. [PubMed] [Google Scholar]
- Comte M., Malnoë A., Cox J. A. Affinity purification of seminalplasmin and characterization of its interaction with calmodulin. Biochem J. 1986 Dec 1;240(2):567–573. doi: 10.1042/bj2400567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishna R., Anderson W. B. Ca2+-induced hydrophobic site on calmodulin: application for purification of calmodulin by phenyl-Sepharose affinity chromatography. Biochem Biophys Res Commun. 1982 Jan 29;104(2):830–836. doi: 10.1016/0006-291x(82)90712-4. [DOI] [PubMed] [Google Scholar]
- Grand R. J., Perry S. V., Weeks R. A. Troponin C-like proteins (calmodulins) from mammalian smooth muscle and other tissues. Biochem J. 1979 Feb 1;177(2):521–529. doi: 10.1042/bj1770521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemric M. E., Chalovich J. M. Effect of caldesmon on the ATPase activity and the binding of smooth and skeletal myosin subfragments to actin. J Biol Chem. 1988 Feb 5;263(4):1878–1885. [PubMed] [Google Scholar]
- Ikebe M., Reardon S. Binding of caldesmon to smooth muscle myosin. J Biol Chem. 1988 Mar 5;263(7):3055–3058. [PubMed] [Google Scholar]
- Keller C. H., Olwin B. B., LaPorte D. C., Storm D. R. Determination of the free-energy coupling for binding of calcium ions and troponin I to calmodulin. Biochemistry. 1982 Jan 5;21(1):156–162. doi: 10.1021/bi00530a027. [DOI] [PubMed] [Google Scholar]
- LaPorte D. C., Keller C. H., Olwin B. B., Storm D. R. Preparation of a fluorescent-labeled derivative of calmodulin which retains its affinity for calmodulin binding proteins. Biochemistry. 1981 Jul 7;20(14):3965–3972. doi: 10.1021/bi00517a004. [DOI] [PubMed] [Google Scholar]
- Mamar-Bachi A., Cox J. A. Quantitative analysis of the free energy coupling in the system calmodulin, calcium, smooth muscle myosin light chain kinase. Cell Calcium. 1987 Dec;8(6):473–482. doi: 10.1016/0143-4160(87)90030-3. [DOI] [PubMed] [Google Scholar]
- Marston S. B. Ca2+ can control vascular smooth-muscle thin filaments without caldesmon phosphorylation. Biochem J. 1986 Jul 15;237(2):605–607. doi: 10.1042/bj2370605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston S. B., Lehman W. Caldesmon is a Ca2+-regulatory component of native smooth-muscle thin filaments. Biochem J. 1985 Nov 1;231(3):517–522. doi: 10.1042/bj2310517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston S. B., Smith C. W. Purification and properties of Ca2+-regulated thin filaments and F-actin from sheep aorta smooth muscle. J Muscle Res Cell Motil. 1984 Oct;5(5):559–575. doi: 10.1007/BF00713261. [DOI] [PubMed] [Google Scholar]
- Marston S. B., Trevett R. M., Walters M. Calcium ion-regulated thin filaments from vascular smooth muscle. Biochem J. 1980 Feb 1;185(2):355–365. doi: 10.1042/bj1850355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston S. Aorta caldesmon inhibits actin activation of thiophosphorylated heavy meromyosin Mg2+-ATPase activity by slowing the rate of product release. FEBS Lett. 1988 Sep 26;238(1):147–150. doi: 10.1016/0014-5793(88)80245-x. [DOI] [PubMed] [Google Scholar]
- Marston S., Pritchard K., Redwood C., Taggart M. Ca2+ regulation of the thin filaments: biochemical mechanism and physiological role. Biochem Soc Trans. 1988 Aug;16(4):494–497. doi: 10.1042/bst0160494. [DOI] [PubMed] [Google Scholar]
- Mills J. S., Walsh M. P., Nemcek K., Johnson J. D. Biologically active fluorescent derivatives of spinach calmodulin that report calmodulin target protein binding. Biochemistry. 1988 Feb 9;27(3):991–996. doi: 10.1021/bi00403a023. [DOI] [PubMed] [Google Scholar]
- Ngai P. K., Walsh M. P. The effects of phosphorylation of smooth-muscle caldesmon. Biochem J. 1987 Jun 1;244(2):417–425. doi: 10.1042/bj2440417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olwin B. B., Edelman A. M., Krebs E. G., Storm D. R. Quantitation of energy coupling between Ca2+, calmodulin, skeletal muscle myosin light chain kinase, and kinase substrates. J Biol Chem. 1984 Sep 10;259(17):10949–10955. [PubMed] [Google Scholar]
- Olwin B. B., Storm D. R. Preparation of fluorescent labeled calmodulins. Methods Enzymol. 1983;102:148–157. doi: 10.1016/s0076-6879(83)02016-9. [DOI] [PubMed] [Google Scholar]
- Pires E. M., Perry S. V. Purification and properties of myosin light-chain kinase from fast skeletal muscle. Biochem J. 1977 Oct 1;167(1):137–146. doi: 10.1042/bj1670137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüegg J. C., Pfitzer G., Zimmer M., Hofmann F. The calmodulin fraction responsible for contraction in an intestinal smooth muscle. FEBS Lett. 1984 May 21;170(2):383–386. doi: 10.1016/0014-5793(84)81349-6. [DOI] [PubMed] [Google Scholar]
- Sellers J. R., Pato M. D., Adelstein R. S. Reversible phosphorylation of smooth muscle myosin, heavy meromyosin, and platelet myosin. J Biol Chem. 1981 Dec 25;256(24):13137–13142. [PubMed] [Google Scholar]
- Smith C. W., Marston S. B. Disassembly and reconstitution of the Ca2+-sensitive thin filaments of vascular smooth muscle. FEBS Lett. 1985 May 6;184(1):115–119. doi: 10.1016/0014-5793(85)80665-7. [DOI] [PubMed] [Google Scholar]
- Smith C. W., Pritchard K., Marston S. B. The mechanism of Ca2+ regulation of vascular smooth muscle thin filaments by caldesmon and calmodulin. J Biol Chem. 1987 Jan 5;262(1):116–122. [PubMed] [Google Scholar]
- Sobieszek A., Bremel R. D. Preparation and properties of vertebrate smooth-muscle myofibrils and actomyosin. Eur J Biochem. 1975 Jun 16;55(1):49–60. doi: 10.1111/j.1432-1033.1975.tb02137.x. [DOI] [PubMed] [Google Scholar]
- Sobue K., Muramoto Y., Fujita M., Kakiuchi S. Purification of a calmodulin-binding protein from chicken gizzard that interacts with F-actin. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5652–5655. doi: 10.1073/pnas.78.9.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]


