Full text
PDF










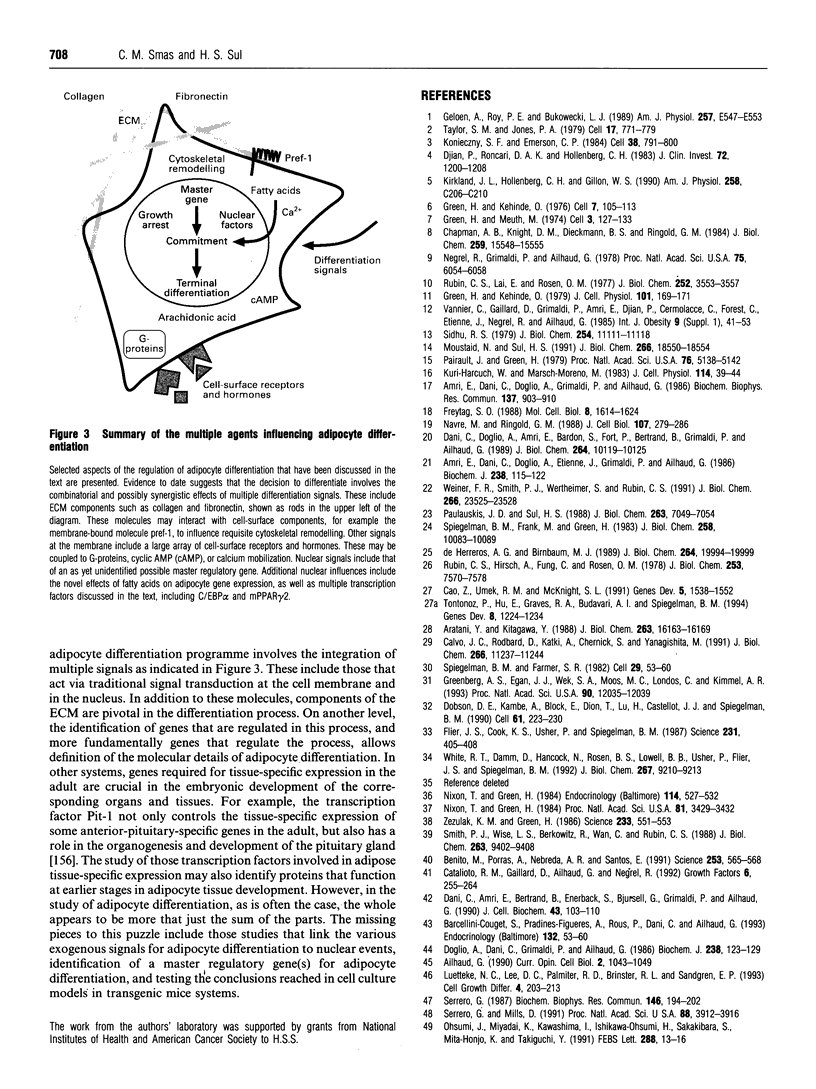


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ailhaud G. Extracellular factors, signalling pathways and differentiation of adipose precursor cells. Curr Opin Cell Biol. 1990 Dec;2(6):1043–1049. doi: 10.1016/0955-0674(90)90154-7. [DOI] [PubMed] [Google Scholar]
- Alexander M. C., Lomanto M., Nasrin N., Ramaika C. Insulin stimulates glyceraldehyde-3-phosphate dehydrogenase gene expression through cis-acting DNA sequences. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5092–5096. doi: 10.1073/pnas.85.14.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amri E. Z., Ailhaud G., Grimaldi P. A. Fatty acids as signal transducing molecules: involvement in the differentiation of preadipose to adipose cells. J Lipid Res. 1994 May;35(5):930–937. [PubMed] [Google Scholar]
- Amri E. Z., Ailhaud G., Grimaldi P. Regulation of adipose cell differentiation. II. Kinetics of induction of the aP2 gene by fatty acids and modulation by dexamethasone. J Lipid Res. 1991 Sep;32(9):1457–1463. [PubMed] [Google Scholar]
- Amri E. Z., Dani C., Doglio A., Etienne J., Grimaldi P., Ailhaud G. Adipose cell differentiation: evidence for a two-step process in the polyamine-dependent Ob1754 clonal line. Biochem J. 1986 Aug 15;238(1):115–122. doi: 10.1042/bj2380115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amri E. Z., Dani C., Doglio A., Grimaldi P., Ailhaud G. Coupling of growth arrest and expression of early markers during adipose conversion of preadipocyte cell lines. Biochem Biophys Res Commun. 1986 Jun 13;137(2):903–910. doi: 10.1016/0006-291x(86)91165-4. [DOI] [PubMed] [Google Scholar]
- Antras J., Hilliou F., Redziniak G., Pairault J. Decreased biosynthesis of actin and cellular fibronectin during adipose conversion of 3T3-F442A cells. Reorganization of the cytoarchitecture and extracellular matrix fibronectin. Biol Cell. 1989;66(3):247–254. [PubMed] [Google Scholar]
- Antras J., Lasnier F., Pairault J. Adipsin gene expression in 3T3-F442A adipocytes is posttranscriptionally down-regulated by retinoic acid. J Biol Chem. 1991 Jan 15;266(2):1157–1161. [PubMed] [Google Scholar]
- Aratani Y., Kitagawa Y. Enhanced synthesis and secretion of type IV collagen and entactin during adipose conversion of 3T3-L1 cells and production of unorthodox laminin complex. J Biol Chem. 1988 Nov 5;263(31):16163–16169. [PubMed] [Google Scholar]
- Barcellini-Couget S., Pradines-Figuères A., Roux P., Dani C., Ailhaud G. The regulation by growth hormone of lipoprotein lipase gene expression is mediated by c-fos protooncogene. Endocrinology. 1993 Jan;132(1):53–60. doi: 10.1210/endo.132.1.8419145. [DOI] [PubMed] [Google Scholar]
- Benito M., Porras A., Nebreda A. R., Santos E. Differentiation of 3T3-L1 fibroblasts to adipocytes induced by transfection of ras oncogenes. Science. 1991 Aug 2;253(5019):565–568. doi: 10.1126/science.1857988. [DOI] [PubMed] [Google Scholar]
- Birkenmeier E. H., Gwynn B., Howard S., Jerry J., Gordon J. I., Landschulz W. H., McKnight S. L. Tissue-specific expression, developmental regulation, and genetic mapping of the gene encoding CCAAT/enhancer binding protein. Genes Dev. 1989 Aug;3(8):1146–1156. doi: 10.1101/gad.3.8.1146. [DOI] [PubMed] [Google Scholar]
- Calvo J. C., Rodbard D., Katki A., Chernick S., Yanagishita M. Differentiation of 3T3-L1 preadipocytes with 3-isobutyl-1-methylxanthine and dexamethasone stimulates cell-associated and soluble chondroitin 4-sulfate proteoglycans. J Biol Chem. 1991 Jun 15;266(17):11237–11244. [PubMed] [Google Scholar]
- Cao Z., Umek R. M., McKnight S. L. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991 Sep;5(9):1538–1552. doi: 10.1101/gad.5.9.1538. [DOI] [PubMed] [Google Scholar]
- Castro-Muñozledo F., Marsch-Moreno M., Beltràn-Langarica A., Kuri-Harcuch W. Commitment of adipocyte differentiation in 3T3 cells is inhibited by retinoic acid, and the expression of lipogenic enzymes is modulated through cytoskeleton stabilization. Differentiation. 1987;36(3):211–219. doi: 10.1111/j.1432-0436.1987.tb00195.x. [DOI] [PubMed] [Google Scholar]
- Catalioto R. M., Gaillard D., Ailhaud G., Négrel R. Terminal differentiation of mouse preadipocyte cells: the mitogenic-adipogenic role of growth hormone is mediated by the protein kinase C signalling pathway. Growth Factors. 1992;6(3):255–264. doi: 10.3109/08977199209026932. [DOI] [PubMed] [Google Scholar]
- Chapman A. B., Knight D. M., Dieckmann B. S., Ringold G. M. Analysis of gene expression during differentiation of adipogenic cells in culture and hormonal control of the developmental program. J Biol Chem. 1984 Dec 25;259(24):15548–15555. [PubMed] [Google Scholar]
- Chawla A., Lazar M. A. Induction of Rev-ErbA alpha, an orphan receptor encoded on the opposite strand of the alpha-thyroid hormone receptor gene, during adipocyte differentiation. J Biol Chem. 1993 Aug 5;268(22):16265–16269. [PubMed] [Google Scholar]
- Chawla A., Lazar M. A. Peroxisome proliferator and retinoid signaling pathways co-regulate preadipocyte phenotype and survival. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1786–1790. doi: 10.1073/pnas.91.5.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Teicher L. C., Kazim D., Pollack R. E., Wise L. S. Commitment of mouse fibroblasts to adipocyte differentiation by DNA transfection. Science. 1989 May 5;244(4904):582–585. doi: 10.1126/science.2470149. [DOI] [PubMed] [Google Scholar]
- Christy R. J., Kaestner K. H., Geiman D. E., Lane M. D. CCAAT/enhancer binding protein gene promoter: binding of nuclear factors during differentiation of 3T3-L1 preadipocytes. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2593–2597. doi: 10.1073/pnas.88.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy R. J., Yang V. W., Ntambi J. M., Geiman D. E., Landschulz W. H., Friedman A. D., Nakabeppu Y., Kelly T. J., Lane M. D. Differentiation-induced gene expression in 3T3-L1 preadipocytes: CCAAT/enhancer binding protein interacts with and activates the promoters of two adipocyte-specific genes. Genes Dev. 1989 Sep;3(9):1323–1335. doi: 10.1101/gad.3.9.1323. [DOI] [PubMed] [Google Scholar]
- Colón-Teicher L., Wise L. S., Martino J. J., Baskin L., Sakoulas G., Pollack R. E., Chen S. Genomic sequences capable of committing mouse and rat fibroblasts to adipogenesis. Nucleic Acids Res. 1993 May 11;21(9):2223–2228. doi: 10.1093/nar/21.9.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J. S., Lucas J. J., Sibley E., Bolanowski M. A., Christy R. J., Kelly T. J., Lane M. D. Expression of the differentiation-induced gene for fatty acid-binding protein is activated by glucocorticoid and cAMP. Proc Natl Acad Sci U S A. 1988 May;85(9):2949–2953. doi: 10.1073/pnas.85.9.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani C., Amri E. Z., Bertrand B., Enerback S., Bjursell G., Grimaldi P., Ailhaud G. Expression and regulation of pOb24 and lipoprotein lipase genes during adipose conversion. J Cell Biochem. 1990 Jun;43(2):103–110. doi: 10.1002/jcb.240430202. [DOI] [PubMed] [Google Scholar]
- Dani C., Doglio A., Amri E. Z., Bardon S., Fort P., Bertrand B., Grimaldi P., Ailhaud G. Cloning and regulation of a mRNA specifically expressed in the preadipose state. J Biol Chem. 1989 Jun 15;264(17):10119–10125. [PubMed] [Google Scholar]
- Darimont C., Gaillard D., Ailhaud G., Negrel R. Terminal differentiation of mouse preadipocyte cells: adipogenic and antimitogenic role of triiodothyronine. Mol Cell Endocrinol. 1993 Dec;98(1):67–73. doi: 10.1016/0303-7207(93)90238-f. [DOI] [PubMed] [Google Scholar]
- Dasarathy Y., Fanburg B. L. Elevation of angiotensin converting enzyme by 3-isobutyl-1-methylxanthine in cultured endothelial cells: a possible role for calmodulin. J Cell Physiol. 1988 Oct;137(1):179–184. doi: 10.1002/jcp.1041370122. [DOI] [PubMed] [Google Scholar]
- Derynck R. Transforming growth factor alpha. Cell. 1988 Aug 26;54(5):593–595. doi: 10.1016/s0092-8674(88)80001-1. [DOI] [PubMed] [Google Scholar]
- Distel R. J., Ro H. S., Rosen B. S., Groves D. L., Spiegelman B. M. Nucleoprotein complexes that regulate gene expression in adipocyte differentiation: direct participation of c-fos. Cell. 1987 Jun 19;49(6):835–844. doi: 10.1016/0092-8674(87)90621-0. [DOI] [PubMed] [Google Scholar]
- Distel R. J., Robinson G. S., Spiegelman B. M. Fatty acid regulation of gene expression. Transcriptional and post-transcriptional mechanisms. J Biol Chem. 1992 Mar 25;267(9):5937–5941. [PubMed] [Google Scholar]
- Djian P., Roncari A. K., Hollenberg C. H. Influence of anatomic site and age on the replication and differentiation of rat adipocyte precursors in culture. J Clin Invest. 1983 Oct;72(4):1200–1208. doi: 10.1172/JCI111075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson D. E., Kambe A., Block E., Dion T., Lu H., Castellot J. J., Jr, Spiegelman B. M. 1-Butyryl-glycerol: a novel angiogenesis factor secreted by differentiating adipocytes. Cell. 1990 Apr 20;61(2):223–230. doi: 10.1016/0092-8674(90)90803-m. [DOI] [PubMed] [Google Scholar]
- Doglio A., Dani C., Grimaldi P., Ailhaud G. Growth hormone regulation of the expression of differentiation-dependent genes in preadipocyte Ob1771 cells. Biochem J. 1986 Aug 15;238(1):123–129. doi: 10.1042/bj2380123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enerbäck S., Ohlsson B. G., Samuelsson L., Bjursell G. Characterization of the human lipoprotein lipase (LPL) promoter: evidence of two cis-regulatory regions, LP-alpha and LP-beta, of importance for the differentiation-linked induction of the LPL gene during adipogenesis. Mol Cell Biol. 1992 Oct;12(10):4622–4633. doi: 10.1128/mcb.12.10.4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flier J. S., Cook K. S., Usher P., Spiegelman B. M. Severely impaired adipsin expression in genetic and acquired obesity. Science. 1987 Jul 24;237(4813):405–408. doi: 10.1126/science.3299706. [DOI] [PubMed] [Google Scholar]
- Fong Y., Lowry S. F. Tumor necrosis factor in the pathophysiology of infection and sepsis. Clin Immunol Immunopathol. 1990 May;55(2):157–170. doi: 10.1016/0090-1229(90)90094-7. [DOI] [PubMed] [Google Scholar]
- Fortini M. E., Artavanis-Tsakonas S. Notch: neurogenesis is only part of the picture. Cell. 1993 Dec 31;75(7):1245–1247. doi: 10.1016/0092-8674(93)90611-s. [DOI] [PubMed] [Google Scholar]
- Freytag S. O. Enforced expression of the c-myc oncogene inhibits cell differentiation by precluding entry into a distinct predifferentiation state in G0/G1. Mol Cell Biol. 1988 Apr;8(4):1614–1624. doi: 10.1128/mcb.8.4.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freytag S. O., Geddes T. J. Reciprocal regulation of adipogenesis by Myc and C/EBP alpha. Science. 1992 Apr 17;256(5055):379–382. doi: 10.1126/science.256.5055.379. [DOI] [PubMed] [Google Scholar]
- Freytag S. O., Paielli D. L., Gilbert J. D. Ectopic expression of the CCAAT/enhancer-binding protein alpha promotes the adipogenic program in a variety of mouse fibroblastic cells. Genes Dev. 1994 Jul 15;8(14):1654–1663. doi: 10.1101/gad.8.14.1654. [DOI] [PubMed] [Google Scholar]
- Fukai F., Iso T., Sekiguchi K., Miyatake N., Tsugita A., Katayama T. An amino-terminal fibronectin fragment stimulates the differentiation of ST-13 preadipocytes. Biochemistry. 1993 Jun 8;32(22):5746–5751. doi: 10.1021/bi00073a004. [DOI] [PubMed] [Google Scholar]
- Furie B., Furie B. C. The molecular basis of blood coagulation. Cell. 1988 May 20;53(4):505–518. doi: 10.1016/0092-8674(88)90567-3. [DOI] [PubMed] [Google Scholar]
- Gaillard D., Négrel R., Lagarde M., Ailhaud G. Requirement and role of arachidonic acid in the differentiation of pre-adipose cells. Biochem J. 1989 Jan 15;257(2):389–397. doi: 10.1042/bj2570389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia de Herreros A., Birnbaum M. J. The acquisition of increased insulin-responsive hexose transport in 3T3-L1 adipocytes correlates with expression of a novel transporter gene. J Biol Chem. 1989 Nov 25;264(33):19994–19999. [PubMed] [Google Scholar]
- Gharbi-Chihi J., Grimaldi P., Torresani J., Ailhaud G. Triiodothyronine and adipose conversion of OB17 preadipocytes : binding to high affinity sites and effects on fatty acid synthetizing and esterifying enzymes. J Recept Res. 1981;2(2):153–173. doi: 10.3109/10799898109039259. [DOI] [PubMed] [Google Scholar]
- Graves R. A., Tontonoz P., Platt K. A., Ross S. R., Spiegelman B. M. Identification of a fat cell enhancer: analysis of requirements for adipose tissue-specific gene expression. J Cell Biochem. 1992 Jul;49(3):219–224. doi: 10.1002/jcb.240490303. [DOI] [PubMed] [Google Scholar]
- Graves R. A., Tontonoz P., Ross S. R., Spiegelman B. M. Identification of a potent adipocyte-specific enhancer: involvement of an NF-1-like factor. Genes Dev. 1991 Mar;5(3):428–437. doi: 10.1101/gad.5.3.428. [DOI] [PubMed] [Google Scholar]
- Green H., Kehinde O. Formation of normally differentiated subcutaneous fat pads by an established preadipose cell line. J Cell Physiol. 1979 Oct;101(1):169–171. doi: 10.1002/jcp.1041010119. [DOI] [PubMed] [Google Scholar]
- Green H., Kehinde O. Spontaneous heritable changes leading to increased adipose conversion in 3T3 cells. Cell. 1976 Jan;7(1):105–113. doi: 10.1016/0092-8674(76)90260-9. [DOI] [PubMed] [Google Scholar]
- Green H., Meuth M. An established pre-adipose cell line and its differentiation in culture. Cell. 1974 Oct;3(2):127–133. doi: 10.1016/0092-8674(74)90116-0. [DOI] [PubMed] [Google Scholar]
- Green H. Terminal differentiation of cultured human epidermal cells. Cell. 1977 Jun;11(2):405–416. doi: 10.1016/0092-8674(77)90058-7. [DOI] [PubMed] [Google Scholar]
- Greenberg A. S., Egan J. J., Wek S. A., Moos M. C., Jr, Londos C., Kimmel A. R. Isolation of cDNAs for perilipins A and B: sequence and expression of lipid droplet-associated proteins of adipocytes. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):12035–12039. doi: 10.1073/pnas.90.24.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grégoire F., De Broux N., Hauser N., Heremans H., Van Damme J., Remacle C. Interferon-gamma and interleukin-1 beta inhibit adipoconversion in cultured rodent preadipocytes. J Cell Physiol. 1992 May;151(2):300–309. doi: 10.1002/jcp.1041510211. [DOI] [PubMed] [Google Scholar]
- Géloën A., Roy P. E., Bukowiecki L. J. Regression of white adipose tissue in diabetic rats. Am J Physiol. 1989 Oct;257(4 Pt 1):E547–E553. doi: 10.1152/ajpendo.1989.257.4.E547. [DOI] [PubMed] [Google Scholar]
- Herrera R., Agarwal S., Walton K., Satterberg B., Distel R. J., Goodman R., Spiegelman B. M., Roberts T. M. A direct role for c-fos in AP-1-dependent gene transcription. Cell Growth Differ. 1990 Oct;1(10):483–490. [PubMed] [Google Scholar]
- Higashiyama S., Abraham J. A., Miller J., Fiddes J. C., Klagsbrun M. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science. 1991 Feb 22;251(4996):936–939. doi: 10.1126/science.1840698. [DOI] [PubMed] [Google Scholar]
- Hunt C. R., Ro J. H., Dobson D. E., Min H. Y., Spiegelman B. M. Adipocyte P2 gene: developmental expression and homology of 5'-flanking sequences among fat cell-specific genes. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3786–3790. doi: 10.1073/pnas.83.11.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahimi A., Bertrand B., Bardon S., Amri E. Z., Grimaldi P., Ailhaud G., Dani C. Cloning of alpha 2 chain of type VI collagen and expression during mouse development. Biochem J. 1993 Jan 1;289(Pt 1):141–147. doi: 10.1042/bj2890141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahimi A., Bonino F., Bardon S., Ailhaud G., Dani C. Essential role of collagens for terminal differentiation of preadipocytes. Biochem Biophys Res Commun. 1992 Sep 30;187(3):1314–1322. doi: 10.1016/0006-291x(92)90446-r. [DOI] [PubMed] [Google Scholar]
- Jones P. L., Schmidhauser C., Bissell M. J. Regulation of gene expression and cell function by extracellular matrix. Crit Rev Eukaryot Gene Expr. 1993;3(2):137–154. [PubMed] [Google Scholar]
- Kaestner K. H., Christy R. J., Lane M. D. Mouse insulin-responsive glucose transporter gene: characterization of the gene and trans-activation by the CCAAT/enhancer binding protein. Proc Natl Acad Sci U S A. 1990 Jan;87(1):251–255. doi: 10.1073/pnas.87.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer S. A., Umesono K., Noonan D. J., Heyman R. A., Evans R. M. Convergence of 9-cis retinoic acid and peroxisome proliferator signalling pathways through heterodimer formation of their receptors. Nature. 1992 Aug 27;358(6389):771–774. doi: 10.1038/358771a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight D. M., Chapman A. B., Navre M., Drinkwater L., Bruno J. J., Ringold G. M. Requirements for triggering of adipocyte differentiation by glucocorticoids and indomethacin. Mol Endocrinol. 1987 Jan;1(1):36–43. doi: 10.1210/mend-1-1-36. [DOI] [PubMed] [Google Scholar]
- Konieczny S. F., Emerson C. P., Jr 5-Azacytidine induction of stable mesodermal stem cell lineages from 10T1/2 cells: evidence for regulatory genes controlling determination. Cell. 1984 Oct;38(3):791–800. doi: 10.1016/0092-8674(84)90274-5. [DOI] [PubMed] [Google Scholar]
- Kuri-Harcuch W., Argüello C., Marsch-Moreno M. Extracellular matrix production by mouse 3T3-F442A cells during adipose differentiation in culture. Differentiation. 1984;28(2):173–178. doi: 10.1111/j.1432-0436.1984.tb00280.x. [DOI] [PubMed] [Google Scholar]
- Kuri-Harcuch W. Differentiation of 3T3-F442A cells into adipocytes is inhibited by retinoic acid. Differentiation. 1982;23(2):164–169. doi: 10.1111/j.1432-0436.1982.tb01279.x. [DOI] [PubMed] [Google Scholar]
- Kuri-Harcuch W., Marsch-Moreno M. DNA synthesis and cell division related to adipose differentiation of 3T3 cells. J Cell Physiol. 1983 Jan;114(1):39–44. doi: 10.1002/jcp.1041140107. [DOI] [PubMed] [Google Scholar]
- Kuri-Harcuch W., Wise L. S., Green H. Interruption of the adipose conversion of 3T3 cells by biotin deficiency: differentiation without triglyceride accumulation. Cell. 1978 May;14(1):53–59. doi: 10.1016/0092-8674(78)90300-8. [DOI] [PubMed] [Google Scholar]
- Li S., Crenshaw E. B., 3rd, Rawson E. J., Simmons D. M., Swanson L. W., Rosenfeld M. G. Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene pit-1. Nature. 1990 Oct 11;347(6293):528–533. doi: 10.1038/347528a0. [DOI] [PubMed] [Google Scholar]
- Lin C. Q., Bissell M. J. Multi-faceted regulation of cell differentiation by extracellular matrix. FASEB J. 1993 Jun;7(9):737–743. doi: 10.1096/fasebj.7.9.8330681. [DOI] [PubMed] [Google Scholar]
- Lin F. T., Lane M. D. Antisense CCAAT/enhancer-binding protein RNA suppresses coordinate gene expression and triglyceride accumulation during differentiation of 3T3-L1 preadipocytes. Genes Dev. 1992 Apr;6(4):533–544. doi: 10.1101/gad.6.4.533. [DOI] [PubMed] [Google Scholar]
- Lin F. T., Lane M. D. CCAAT/enhancer binding protein alpha is sufficient to initiate the 3T3-L1 adipocyte differentiation program. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8757–8761. doi: 10.1073/pnas.91.19.8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. T., MacDougald O. A., Diehl A. M., Lane M. D. A 30-kDa alternative translation product of the CCAAT/enhancer binding protein alpha message: transcriptional activator lacking antimitotic activity. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9606–9610. doi: 10.1073/pnas.90.20.9606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luetteke N. C., Lee D. C., Palmiter R. D., Brinster R. L., Sandgren E. P. Regulation of fat and muscle development by transforming growth factor alpha in transgenic mice and in cultured cells. Cell Growth Differ. 1993 Mar;4(3):203–213. [PubMed] [Google Scholar]
- Mauro V. P., Wood I. C., Krushel L., Crossin K. L., Edelman G. M. Cell adhesion alters gene transcription in chicken embryo brain cells and mouse embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1994 Mar 29;91(7):2868–2872. doi: 10.1073/pnas.91.7.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEver R. P. Leukocyte-endothelial cell interactions. Curr Opin Cell Biol. 1992 Oct;4(5):840–849. doi: 10.1016/0955-0674(92)90109-p. [DOI] [PubMed] [Google Scholar]
- McGehee R. E., Jr, Ron D., Brasier A. R., Habener J. F. Differentiation-specific element: a cis-acting developmental switch required for the sustained transcriptional expression of the angiotensinogen gene during hormonal-induced differentiation of 3T3-L1 fibroblasts to adipocytes. Mol Endocrinol. 1993 Apr;7(4):551–560. doi: 10.1210/mend.7.4.7684818. [DOI] [PubMed] [Google Scholar]
- McKeon C., Pham T. Transactivation of the human insulin receptor gene by the CAAT/enhancer binding protein. Biochem Biophys Res Commun. 1991 Jan 31;174(2):721–728. doi: 10.1016/0006-291x(91)91477-t. [DOI] [PubMed] [Google Scholar]
- Moustaïd N., Sakamoto K., Clarke S., Beyer R. S., Sul H. S. Regulation of fatty acid synthase gene transcription. Sequences that confer a positive insulin effect and differentiation-dependent expression in 3T3-L1 preadipocytes are present in the 332 bp promoter. Biochem J. 1993 Jun 15;292(Pt 3):767–772. doi: 10.1042/bj2920767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustaïd N., Sul H. S. Regulation of expression of the fatty acid synthase gene in 3T3-L1 cells by differentiation and triiodothyronine. J Biol Chem. 1991 Oct 5;266(28):18550–18554. [PubMed] [Google Scholar]
- Murphy-Ullrich J. E., Schultz-Cherry S., Hök M. Transforming growth factor-beta complexes with thrombospondin. Mol Biol Cell. 1992 Feb;3(2):181–188. doi: 10.1091/mbc.3.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAPOLITANO L. THE DIFFERENTIATION OF WHITE ADIPOSE CELLS. AN ELECTRON MICROSCOPE STUDY. J Cell Biol. 1963 Sep;18:663–679. doi: 10.1083/jcb.18.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navre M., Ringold G. M. A growth factor-repressible gene associated with protein kinase C-mediated inhibition of adipocyte differentiation. J Cell Biol. 1988 Jul;107(1):279–286. doi: 10.1083/jcb.107.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navre M., Ringold G. M. Differential effects of fibroblast growth factor and tumor promoters on the initiation and maintenance of adipocyte differentiation. J Cell Biol. 1989 Oct;109(4 Pt 1):1857–1863. doi: 10.1083/jcb.109.4.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuberg M., Adamkiewicz J., Hunter J. B., Müller R. A Fos protein containing the Jun leucine zipper forms a homodimer which binds to the AP1 binding site. Nature. 1989 Sep 21;341(6239):243–245. doi: 10.1038/341243a0. [DOI] [PubMed] [Google Scholar]
- Ninomiya-Tsuji J., Torti F. M., Ringold G. M. Tumor necrosis factor-induced c-myc expression in the absence of mitogenesis is associated with inhibition of adipocyte differentiation. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9611–9615. doi: 10.1073/pnas.90.20.9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon B. T., Green H. Growth hormone promotes the differentiation of myoblasts and preadipocytes generated by azacytidine treatment of 10T1/2 cells. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3429–3432. doi: 10.1073/pnas.81.11.3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon T., Green H. Contribution of growth hormone to the adipogenic activity of serum. Endocrinology. 1984 Feb;114(2):527–532. doi: 10.1210/endo-114-2-527. [DOI] [PubMed] [Google Scholar]
- Négrel R., Gaillard D., Ailhaud G. Prostacyclin as a potent effector of adipose-cell differentiation. Biochem J. 1989 Jan 15;257(2):399–405. doi: 10.1042/bj2570399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Négrel R., Grimaldi P., Ailhaud G. Establishment of preadipocyte clonal line from epididymal fat pad of ob/ob mouse that responds to insulin and to lipolytic hormones. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6054–6058. doi: 10.1073/pnas.75.12.6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsumi J., Miyadai K., Kawashima I., Ishikawa-Ohsumi H., Sakakibara S., Mita-Honjo K., Takiguchi Y. Adipogenesis inhibitory factor. A novel inhibitory regulator of adipose conversion in bone marrow. FEBS Lett. 1991 Aug 19;288(1-2):13–16. doi: 10.1016/0014-5793(91)80991-b. [DOI] [PubMed] [Google Scholar]
- Pairault J., Green H. A study of the adipose conversion of suspended 3T3 cells by using glycerophosphate dehydrogenase as differentiation marker. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5138–5142. doi: 10.1073/pnas.76.10.5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pairault J., Lasnier F. Dihydrocytochalasin B promotes adipose conversion of 3T3 cells. Biol Cell. 1987;61(3):149–154. doi: 10.1111/j.1768-322x.1987.tb00581.x. [DOI] [PubMed] [Google Scholar]
- Pairault J., Quignard-Boulange A., Dugail I., Lasnier F. Differential effects of retinoic acid upon early and late events in adipose conversion of 3T3 preadipocytes. Exp Cell Res. 1988 Jul;177(1):27–36. doi: 10.1016/0014-4827(88)90022-5. [DOI] [PubMed] [Google Scholar]
- Parsons W. J., Stiles G. L. Methylxanthines as adenosine receptor antagonists. Ann Intern Med. 1985 Oct;103(4):643–643. doi: 10.7326/0003-4819-103-4-643_1. [DOI] [PubMed] [Google Scholar]
- Paulauskis J. D., Sul H. S. Cloning and expression of mouse fatty acid synthase and other specific mRNAs. Developmental and hormonal regulation in 3T3-L1 cells. J Biol Chem. 1988 May 25;263(15):7049–7054. [PubMed] [Google Scholar]
- Petruschke T., Hauner H. Tumor necrosis factor-alpha prevents the differentiation of human adipocyte precursor cells and causes delipidation of newly developed fat cells. J Clin Endocrinol Metab. 1993 Mar;76(3):742–747. doi: 10.1210/jcem.76.3.8445033. [DOI] [PubMed] [Google Scholar]
- Plowman G. D., Green J. M., McDonald V. L., Neubauer M. G., Disteche C. M., Todaro G. J., Shoyab M. The amphiregulin gene encodes a novel epidermal growth factor-related protein with tumor-inhibitory activity. Mol Cell Biol. 1990 May;10(5):1969–1981. doi: 10.1128/mcb.10.5.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauscher F. J., 3rd, Sambucetti L. C., Curran T., Distel R. J., Spiegelman B. M. Common DNA binding site for Fos protein complexes and transcription factor AP-1. Cell. 1988 Feb 12;52(3):471–480. doi: 10.1016/s0092-8674(88)80039-4. [DOI] [PubMed] [Google Scholar]
- Ron D., Brasier A. R., McGehee R. E., Jr, Habener J. F. Tumor necrosis factor-induced reversal of adipocytic phenotype of 3T3-L1 cells is preceded by a loss of nuclear CCAAT/enhancer binding protein (C/EBP). J Clin Invest. 1992 Jan;89(1):223–233. doi: 10.1172/JCI115566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D., Habener J. F. CHOP, a novel developmentally regulated nuclear protein that dimerizes with transcription factors C/EBP and LAP and functions as a dominant-negative inhibitor of gene transcription. Genes Dev. 1992 Mar;6(3):439–453. doi: 10.1101/gad.6.3.439. [DOI] [PubMed] [Google Scholar]
- Ross S. R., Graves R. A., Greenstein A., Platt K. A., Shyu H. L., Mellovitz B., Spiegelman B. M. A fat-specific enhancer is the primary determinant of gene expression for adipocyte P2 in vivo. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9590–9594. doi: 10.1073/pnas.87.24.9590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin C. S., Hirsch A., Fung C., Rosen O. M. Development of hormone receptors and hormonal responsiveness in vitro. Insulin receptors and insulin sensitivity in the preadipocyte and adipocyte forms of 3T3-L1 cells. J Biol Chem. 1978 Oct 25;253(20):7570–7578. [PubMed] [Google Scholar]
- Rubin C. S., Lai E., Rosen O. M. Acquisition of increased hormone sensitivity during in vitro adipocyte development. J Biol Chem. 1977 May 25;252(10):3554–3557. [PubMed] [Google Scholar]
- Russo J. J., Manuli M. A., Ismail-Beigi F., Sweadner K. J., Edelman I. S. Na(+)-K(+)-ATPase in adipocyte differentiation in culture. Am J Physiol. 1990 Dec;259(6 Pt 1):C968–C977. doi: 10.1152/ajpcell.1990.259.6.C968. [DOI] [PubMed] [Google Scholar]
- Sadowski H. B., Wheeler T. T., Young D. A. Gene expression during 3T3-L1 adipocyte differentiation. Characterization of initial responses to the inducing agents and changes during commitment to differentiation. J Biol Chem. 1992 Mar 5;267(7):4722–4731. [PubMed] [Google Scholar]
- Safonova I., Darimont C., Amri E. Z., Grimaldi P., Ailhaud G., Reichert U., Shroot B. Retinoids are positive effectors of adipose cell differentiation. Mol Cell Endocrinol. 1994 Sep;104(2):201–211. doi: 10.1016/0303-7207(94)90123-6. [DOI] [PubMed] [Google Scholar]
- Samuelsson L., Strömberg K., Vikman K., Bjursell G., Enerbäck S. The CCAAT/enhancer binding protein and its role in adipocyte differentiation: evidence for direct involvement in terminal adipocyte development. EMBO J. 1991 Dec;10(12):3787–3793. doi: 10.1002/j.1460-2075.1991.tb04948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saye J., Lynch K. R., Peach M. J. Changes in angiotensinogen messenger RNA in differentiating 3T3-F442A adipocytes. Hypertension. 1990 Jun;15(6 Pt 2):867–871. doi: 10.1161/01.hyp.15.6.867. [DOI] [PubMed] [Google Scholar]
- Serrero G. EGF inhibits the differentiation of adipocyte precursors in primary cultures. Biochem Biophys Res Commun. 1987 Jul 15;146(1):194–202. doi: 10.1016/0006-291x(87)90710-8. [DOI] [PubMed] [Google Scholar]
- Serrero G., Lepak N. M., Goodrich S. P. Prostaglandin F2 alpha inhibits the differentiation of adipocyte precursors in primary culture. Biochem Biophys Res Commun. 1992 Mar 16;183(2):438–442. doi: 10.1016/0006-291x(92)90500-k. [DOI] [PubMed] [Google Scholar]
- Serrero G., Mills D. Decrease in transforming growth factor beta 1 binding during differentiation of rat adipocyte precursors in primary culture. Cell Growth Differ. 1991 Mar;2(3):173–178. [PubMed] [Google Scholar]
- Serrero G., Mills D. Physiological role of epidermal growth factor on adipose tissue development in vivo. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3912–3916. doi: 10.1073/pnas.88.9.3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu R. S. Two-dimensional electrophoretic analyses of proteins synthesized during differentiation of 3T3-L1 preadipocytes. J Biol Chem. 1979 Nov 10;254(21):11111–11118. [PubMed] [Google Scholar]
- Smas C. M., Green D., Sul H. S. Structural characterization and alternate splicing of the gene encoding the preadipocyte EGF-like protein pref-1. Biochemistry. 1994 Aug 9;33(31):9257–9265. doi: 10.1021/bi00197a029. [DOI] [PubMed] [Google Scholar]
- Smas C. M., Sul H. S. Pref-1, a protein containing EGF-like repeats, inhibits adipocyte differentiation. Cell. 1993 May 21;73(4):725–734. doi: 10.1016/0092-8674(93)90252-l. [DOI] [PubMed] [Google Scholar]
- Smith P. J., Wise L. S., Berkowitz R., Wan C., Rubin C. S. Insulin-like growth factor-I is an essential regulator of the differentiation of 3T3-L1 adipocytes. J Biol Chem. 1988 Jul 5;263(19):9402–9408. [PubMed] [Google Scholar]
- Spiegelman B. M., Farmer S. R. Decreases in tubulin and actin gene expression prior to morphological differentiation of 3T3 adipocytes. Cell. 1982 May;29(1):53–60. doi: 10.1016/0092-8674(82)90089-7. [DOI] [PubMed] [Google Scholar]
- Spiegelman B. M., Ginty C. A. Fibronectin modulation of cell shape and lipogenic gene expression in 3T3-adipocytes. Cell. 1983 Dec;35(3 Pt 2):657–666. doi: 10.1016/0092-8674(83)90098-3. [DOI] [PubMed] [Google Scholar]
- Spiegelman B. M., Green H. Control of specific protein biosynthesis during the adipose conversion of 3T3 cells. J Biol Chem. 1980 Sep 25;255(18):8811–8818. [PubMed] [Google Scholar]
- Spiegelman B. M., Green H. Cyclic AMP-mediated control of lipogenic enzyme synthesis during adipose differentiation of 3T3 cells. Cell. 1981 May;24(2):503–510. doi: 10.1016/0092-8674(81)90341-x. [DOI] [PubMed] [Google Scholar]
- Stein B., Cogswell P. C., Baldwin A. S., Jr Functional and physical associations between NF-kappa B and C/EBP family members: a Rel domain-bZIP interaction. Mol Cell Biol. 1993 Jul;13(7):3964–3974. doi: 10.1128/mcb.13.7.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens J. M., Pekala P. H. Transcriptional repression of the GLUT4 and C/EBP genes in 3T3-L1 adipocytes by tumor necrosis factor-alpha. J Biol Chem. 1991 Nov 15;266(32):21839–21845. [PubMed] [Google Scholar]
- Stone R. L., Bernlohr D. A. The molecular basis for inhibition of adipose conversion of murine 3T3-L1 cells by retinoic acid. Differentiation. 1990 Nov;45(2):119–127. doi: 10.1111/j.1432-0436.1990.tb00465.x. [DOI] [PubMed] [Google Scholar]
- Streuli C. H., Bailey N., Bissell M. J. Control of mammary epithelial differentiation: basement membrane induces tissue-specific gene expression in the absence of cell-cell interaction and morphological polarity. J Cell Biol. 1991 Dec;115(5):1383–1395. doi: 10.1083/jcb.115.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H. L., Malbon C. C., Wang H. Y. Increased expression of Gi alpha 2 in mouse embryo stem cells promotes terminal differentiation to adipocytes. Am J Physiol. 1993 Dec;265(6 Pt 1):C1729–C1735. doi: 10.1152/ajpcell.1993.265.6.C1729. [DOI] [PubMed] [Google Scholar]
- Swick A. G., Lane M. D. Identification of a transcriptional repressor down-regulated during preadipocyte differentiation. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):7895–7899. doi: 10.1073/pnas.89.17.7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapscott S. J., Davis R. L., Thayer M. J., Cheng P. F., Weintraub H., Lassar A. B. MyoD1: a nuclear phosphoprotein requiring a Myc homology region to convert fibroblasts to myoblasts. Science. 1988 Oct 21;242(4877):405–411. doi: 10.1126/science.3175662. [DOI] [PubMed] [Google Scholar]
- Taylor S. M., Jones P. A. Multiple new phenotypes induced in 10T1/2 and 3T3 cells treated with 5-azacytidine. Cell. 1979 Aug;17(4):771–779. doi: 10.1016/0092-8674(79)90317-9. [DOI] [PubMed] [Google Scholar]
- Teboul M., Bismuth J., Gharbi-Chihi J., Vallette A., Bonne J., Ghiringhelli O., Torresani J. Retinoic acid decreases nuclear triiodothyronine receptor expression and impairs an early step of adipose differentiation in the thyroid hormone-sensitive mouse Ob 17 preadipocyte cell line. Endocrinology. 1992 Mar;130(3):1475–1482. doi: 10.1210/endo.130.3.1311241. [DOI] [PubMed] [Google Scholar]
- Thiery J. P., Boyer B. The junction between cytokines and cell adhesion. Curr Opin Cell Biol. 1992 Oct;4(5):782–792. doi: 10.1016/0955-0674(92)90101-h. [DOI] [PubMed] [Google Scholar]
- Tontonoz P., Hu E., Graves R. A., Budavari A. I., Spiegelman B. M. mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994 May 15;8(10):1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- Tontonoz P., Hu E., Spiegelman B. M. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994 Dec 30;79(7):1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Tontonoz P., Kim J. B., Graves R. A., Spiegelman B. M. ADD1: a novel helix-loop-helix transcription factor associated with adipocyte determination and differentiation. Mol Cell Biol. 1993 Aug;13(8):4753–4759. doi: 10.1128/mcb.13.8.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torti F. M., Dieckmann B., Beutler B., Cerami A., Ringold G. M. A macrophage factor inhibits adipocyte gene expression: an in vitro model of cachexia. Science. 1985 Aug 30;229(4716):867–869. doi: 10.1126/science.3839597. [DOI] [PubMed] [Google Scholar]
- Umek R. M., Friedman A. D., McKnight S. L. CCAAT-enhancer binding protein: a component of a differentiation switch. Science. 1991 Jan 18;251(4991):288–292. doi: 10.1126/science.1987644. [DOI] [PubMed] [Google Scholar]
- Umezawa A., Hata J. Expression of gap-junctional protein (connexin 43 or alpha 1 gap junction) is down-regulated at the transcriptional level during adipocyte differentiation of H-1/A marrow stromal cells. Cell Struct Funct. 1992 Jun;17(3):177–184. doi: 10.1247/csf.17.177. [DOI] [PubMed] [Google Scholar]
- Vallejo M., Ron D., Miller C. P., Habener J. F. C/ATF, a member of the activating transcription factor family of DNA-binding proteins, dimerizes with CAAT/enhancer-binding proteins and directs their binding to cAMP response elements. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4679–4683. doi: 10.1073/pnas.90.10.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannier C., Gaillard D., Grimaldi P., Amri E. Z., Djian P., Cermolacce C., Forest C., Etienne J., Negrel R., Ailhaud G. Adipose conversion of ob17 cells and hormone-related events. Int J Obes. 1985;9 (Suppl 1):41–53. [PubMed] [Google Scholar]
- Vassaux G., Gaillard D., Ailhaud G., Négrel R. Prostacyclin is a specific effector of adipose cell differentiation. Its dual role as a cAMP- and Ca(2+)-elevating agent. J Biol Chem. 1992 Jun 5;267(16):11092–11097. [PubMed] [Google Scholar]
- Wang H. Y., Watkins D. C., Malbon C. C. Antisense oligodeoxynucleotides to GS protein alpha-subunit sequence accelerate differentiation of fibroblasts to adipocytes. Nature. 1992 Jul 23;358(6384):334–337. doi: 10.1038/358334a0. [DOI] [PubMed] [Google Scholar]
- Weiner F. R., Shah A., Smith P. J., Rubin C. S., Zern M. A. Regulation of collagen gene expression in 3T3-L1 cells. Effects of adipocyte differentiation and tumor necrosis factor alpha. Biochemistry. 1989 May 2;28(9):4094–4099. doi: 10.1021/bi00435a070. [DOI] [PubMed] [Google Scholar]
- Weiner F. R., Smith P. J., Wertheimer S., Rubin C. S. Regulation of gene expression by insulin and tumor necrosis factor alpha in 3T3-L1 cells. Modulation of the transcription of genes encoding acyl-CoA synthetase and stearoyl-CoA desaturase-1. J Biol Chem. 1991 Dec 15;266(35):23525–23528. [PubMed] [Google Scholar]
- Wenz H. M., Hinck L., Cannon P., Navre M., Ringold G. M. Reduced expression of AP27 protein, the product of a growth factor-repressible gene, is associated with diminished adipocyte differentiation. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):1065–1069. doi: 10.1073/pnas.89.3.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. T., Damm D., Hancock N., Rosen B. S., Lowell B. B., Usher P., Flier J. S., Spiegelman B. M. Human adipsin is identical to complement factor D and is expressed at high levels in adipose tissue. J Biol Chem. 1992 May 5;267(13):9210–9213. [PubMed] [Google Scholar]
- Wilkison W. O., Min H. Y., Claffey K. P., Satterberg B. L., Spiegelman B. M. Control of the adipsin gene in adipocyte differentiation. Identification of distinct nuclear factors binding to single- and double-stranded DNA. J Biol Chem. 1990 Jan 5;265(1):477–482. [PubMed] [Google Scholar]
- Williams I. H., Polakis S. E. Differentiation of 3T3-L1 fibroblasts to adipocytes. The effect of indomethacin, prostaglandin E1 and cyclic AMP on the process of differentiation. Biochem Biophys Res Commun. 1977 Jul 11;77(1):175–186. doi: 10.1016/s0006-291x(77)80180-0. [DOI] [PubMed] [Google Scholar]
- Williams P. M., Chang D. J., Danesch U., Ringold G. M., Heller R. A. CCAAT/enhancer binding protein expression is rapidly extinguished in TA1 adipocyte cells treated with tumor necrosis factor. Mol Endocrinol. 1992 Jul;6(7):1135–1141. doi: 10.1210/mend.6.7.1508226. [DOI] [PubMed] [Google Scholar]
- Wong W. T., Nick H. S., Frost S. C. Regulation of annexin I in adipogenesis: cAMP-independent action of methylisobutylxanthine. Am J Physiol. 1992 Jan;262(1 Pt 1):C91–C97. doi: 10.1152/ajpcell.1992.262.1.C91. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y., Mann D. M., Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature. 1990 Jul 19;346(6281):281–284. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]
- Zezulak K. M., Green H. The generation of insulin-like growth factor-1--sensitive cells by growth hormone action. Science. 1986 Aug 1;233(4763):551–553. doi: 10.1126/science.3726546. [DOI] [PubMed] [Google Scholar]