Abstract
Molds pose a severe challenge to agriculture because they cause very large crop losses. For this reason, synthetic fungicides have been used for a long time. Without adequate protection against pests and various pathogens, crop losses could be as high as 30–40%. However, concerns mainly about the environmental impact of synthetic antifungals and human health risk have prompted a search for natural alternatives. But do natural remedies only have advantages? This article reviews the current state of knowledge on the use of antifungal substances in agriculture to protect seeds against phytopathogens. The advantages and disadvantages of using both synthetic and natural fungicides to protect cereal grains were discussed, indicating specific examples and mechanisms of action. The possibilities of an integrated control approach, combining cultural, biological, and chemical methods are described, constituting a holistic strategy for sustainable mold management in the grain industry.
Keywords: chemical fungicides, biocontrol agents, integrated control, seed preservation
1. Introduction
Molds are one of the most difficult pathogenic microorganisms to combat during crop production, from the sowing stage through the development of young plants to harvesting and storage. Grain can become infected with fungi in the field when the plant is ripening. The infection may come from the mother plant or from external sources, such as insects or wind. Commonly isolated genera belonging to field molds include Fusarium, Alternaria, and Cladosporium. These genera cause damage or death to embryos, are potential producers of mycotoxins, and contribute to disease in new seedlings if infected seed is selected for sowing. Storage molds are genera that invade seeds during storage in warehouses. They include, in particular, the genera Aspergillus and Penicillium. These genera reduce the germination capacity of grain, produce mycotoxins, and at high humidity contribute to the spontaneous heating of stored grains, which drastically reduces the quality of the seed material. Fungi can also attack grain immediately after sowing, sticking to the surface or entering into the seed tissue. They then cause the weakening or death of embryos, which contributes to large crop losses at the very beginning of the cultivation process. Infection of crops leads not only to serious crop losses but, in the case of mycotoxin accumulation also causes health problems in humans and animals.
There are several ways to protect plants from fungal attack. One of the most effective methods is seed dressing. Seed dressing protects the plant from the earliest stages of growth, which often translates into fewer mold infections in the adult plant. For this reason, plant protection products have been used in agriculture for many years. However, most conventional plant protection products contain high levels of synthetic chemical compounds, which may be toxic. To protect the environment and human health, the use of synthetic compounds is increasingly limited. For example, in 2020, European Union countries adopted a “farm to fork” strategy aimed at promoting sustainable agriculture and healthy food. This strategy has contributed to restricting the substances that can be used on crops. As more and more chemical compounds have been withdrawn from European markets, new more natural plant protection products that fit into the green deal strategy are being sought. Microbiological biopreparations offer one possible alternative to chemical plant protection products.
This article reviews the current state of the art regarding the use of antifungal substances in agriculture for protecting seeds against phytopathogens. It discusses the benefits and disadvantages of both synthetic and natural fungicides for cereal grain protection.
2. Bibliometric Data Mapping and Clustering
Bibliographic records on chemical and natural fungicides used for the protection of cereal grains were retrieved from the Scopus database. The following reference word combinations were used to search for relevant bibliographic records based on keywords, titles, and abstracts: ‘fungicides AND cereal AND grains’ and ‘biocontrol AND cereal AND grains’. The AND hyphen between reference words was used to search only for publications related to the described scientific area. No time period was indicated, but the oldest publication in the Scopus database for the combination of the words ‘fungicides AND cereal AND grains’ was from 1959, while the latest was from 2024 (data was searched in April 2024). In total, 349 records were found, 59 of which were published up to the year 2000, while 290 were published in the last 24 years. In the years 1963–1972, no records containing these keywords were found.
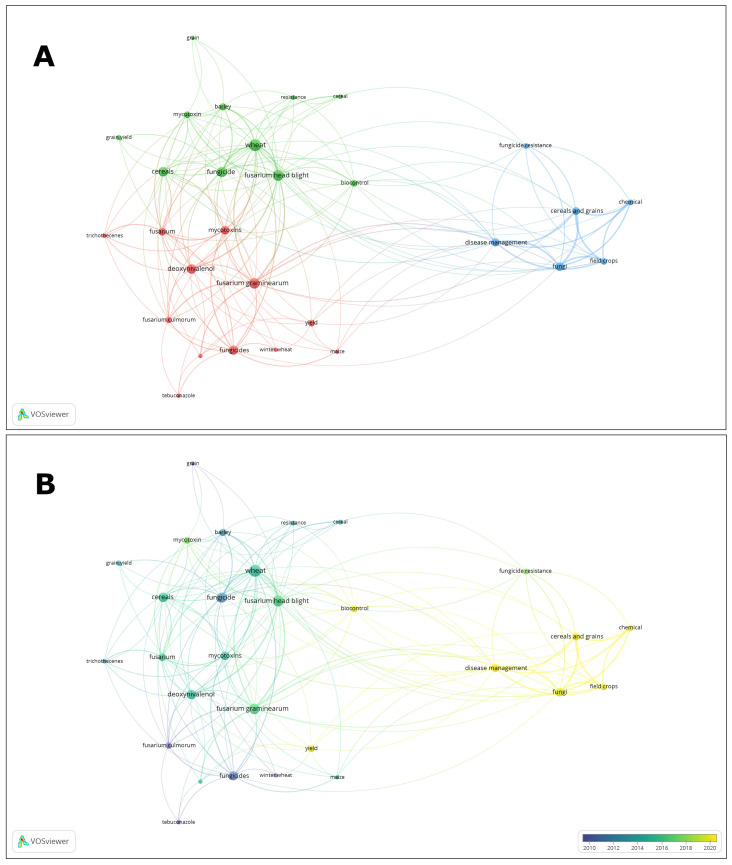
VOSviewer version 1.6.20 software (available at www.vosviewer.com, accessed on 30 May 2024) was used to visualize scientific landscape maps. VOSviewer distinguished three clusters, two of which were strongly related to the terms ‘biocontrol’ and ‘disease management’ (Figure 1A). It was in these areas that the publications from 2018 were focused (Figure 1B). The content of the papers included the mechanisms of fungicide action, based on information provided by the Fungicide Resistance Action Committee (FRAC). The advantages and disadvantages of synthetic agents and natural solutions were also discussed, including their impact on the environment, together with the possibility of simultaneous use of synthetic and natural substances using Integrated Pest and Disease Management (IDPM) strategies.
Figure 1.
Term map based on keywords ‘fungicides’ and ‘cereal grains’. The colors on the map indicate terms belonging to different clusters (A) or the year of publication (B). The lengths of the lines correspond to the interrelationships between the terms. Bubble size presents the number of papers in the database. Bubble proximity presents frequency of co-occurrence of phrases in the same papers.
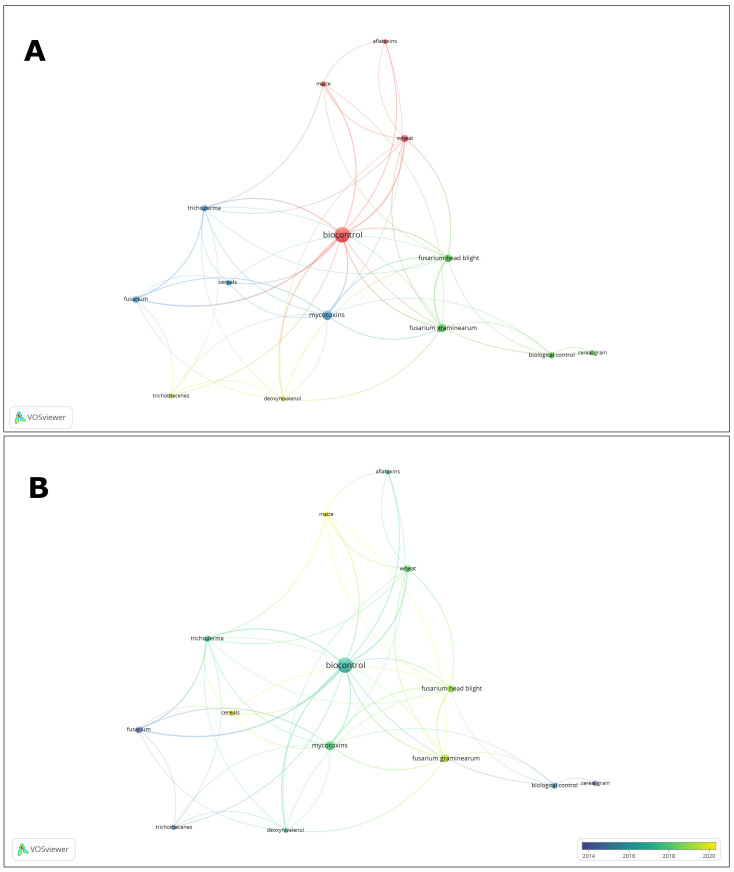
For the combination of the words ‘biocontrol AND cereal AND grains’, 156 documents were found. Interestingly, the first record was not published until 1998, and the number of publications increased after 2018. Despite the lower number of publications than were found for the keywords ‘fungicides’ and ‘cereal grains’, four clusters were distinguished, indicating strong connections between the terms ‘biocontrol’, ‘mycotoxins’, and ‘Fusarium graminearum’ (Figure 2A,B).
Figure 2.
Term map based on keywords ‘biocontrol’ and ‘cereal grains’. The colors on the map indicate terms belonging to different clusters (A) or the year of publication (B). The lengths of the lines correspond to the interrelationships between the terms. Bubble size presents the number of papers in the database. Bubble proximity presents frequency of co-occurrence of phrases in the same papers.
3. Synthetic Fungicides
Fungicides are chemical substances used to combat fungi that infect plants. In agriculture, they are used to protect field crops: cereals, vegetables, fruit, ornamental plants, as well as greenhouse crops. Their task is to eliminate or inhibit the development of fungi and their spores. Fungicides can help to combat fungal infections during the sowing stage and during plant growth, improving the quality and durability of cultivated crops, as well as increasing harvest efficiency [1,2,3,4,5].
3.1. Short History
Plant pathogens such as fungi are often associated with significant losses during crop production, in both pre- and post-harvest scenarios. Over the past 100 years, the use of fungicides has contributed to reducing infections significantly [6], which has led to increased agricultural production efficiency [7]. Until the 1940s, the substances most commonly used to control diseases such as blights, mildews, and blasts were inorganic compounds of copper, which had been known since the mid-1700s [8]. In the early 1920s, the idea of using organic mercury compounds as a seed treatment emerged [9]. However, due to the high toxicity of mercury, this conservation method was abandoned in the 1960s, as it posed a danger to human and animal health as well as to the environment [10]. Further research led to the development of synthetic organic compounds in the form of dithiocarbamates in 1934, which were soon introduced on the market. Over the next 30–40 years, other compounds including phthalimides, fentins, and guanidines, were introduced [11]. These multi-site contact fungicides were characterized by low specificity and were limited to protection at the surface [12]. In the 1970s, the first systemic fungicide, carboxin (carboxamides), appeared, later followed by benzimidazoles, thiophanates, and morpholines [13]. In contrast to multi-site fungicides, systemic fungicides are site-specific, act internally to hinder infections, and can be used at much lower concentrations [7]. Since the turn of the 21st century, many new classes of fungicides have been developed. The most widely used are demethylation inhibitors (DMI), succinate dehydrogenase inhibitors (SDHI), carboxylic acid amides (CAA), and strobilurins [14]. These substances are most often paired with multi-site inhibitors to prevent pathogens from developing resistance due to the high specificity of the new fungicides [7]. Other recent approaches involve the use of compounds that activate the host’s defense system, such as physcion, probenazole, acibenzolar-S methyl, or dichlobentiazox [15,16].
Research on new products is driven by many factors, such as the development of resistance to previous generations of active substances, changes in legislation regarding the requirements for products, and demand from farmers themselves [17,18]. The type of substance used, the strain of the pathogen, and the method of applying the protective agents each have a large impact on the development of resistance [19,20,21]. Many of the fungicides currently in use are partially ineffective due to overuse or incorrect use [22]. For example, excessive use of triazoles causes the rapid development of resistance, the mechanism of action of strobilurins can be easily ignored by fungal cells that develop additional electron transport pathways, and the misuse of morpholines can lead to the development of fungal resistance. Specifically, morpholines inhibit the synthesis of ergosterol, an essential component of fungal cell membranes, by blocking the reduction reaction in the sterol biosynthesis pathway [1,19,23]. As well as pairing different substances, many other procedures are also used to extend the life (protective capacity) of these products as much as possible. These methods include the use of crop rotation, intercropping, or covering crops [24]. There is also legislative pressure to find innovative solutions. Directives from the European Union clearly indicate the parameters that should be characteristic of products intended for use in the EU, placing particular emphasis on their toxicological aspects in relation to both the environment and humans [25]. It is also important to remember that the substances used should have as negligible an impact on the plant as possible, so as not to cause additional yield losses. Despite this, some permitted substances can cause reduced transpiration, CO2 assimilation, and decreased production of biomass or sterols [1].
3.2. Cereal Crops around the Globe
Crops cover about 47.5 million square kilometers of the globe’s surface. Without adequate protection against pests and various pathogens, crop losses could be as high as 30–40% [26,27], which would most likely cause famine in many regions on earth. Various pesticides are used to prevent crop losses from occurring, including herbicides, insecticides, and fungicides. In 2022, it was reported that 117.9 kg/ha of pesticides were used for protective purposes in agriculture, of which 40% were fungicides. In the four examined countries/regions (Table 1) of the world, the EU had the highest usage of pesticides at 38.8 kg/ha, of which 19.8 kg/ha were fungicides. In the other countries/regions, pesticide use was notably lower [28]. According to FAO data from 2022 concerning the production of selected cereals (wheat, rye, barley, oats, and corn), China is the largest overall producer of corn (277.4 Mt), wheat (137.7 Mt), rye (0.5 Mt), barley (2 Mt), and oats (0.6 Mt), both in terms of the amounts produced and the area of cultivation. The USA produces similar amounts to China of rye, barley, and oats, but three times less wheat (44.9 Mt). Data collected from 27 EU countries show the highest values for cereal production related to wheat (134.3 Mt), barley (52 Mt), rye (7.5 Mt), and oats (7.5 Mt). Corn production in the EU (27 countries) is incomparably lower than in the other mentioned countries/regions, at 8.8 Mt. In Brazil, the quantities of cereals produced range from 10.3–11.2 Mt for wheat and rye and 0.5–1.6 Mt for barley and oats, while corn reached 109.4 Mt. China produced the largest quantity of wheat, at 137.7 Mt. The EU (27 countries) produced the largest quantities of rye (7.5 Mt), barley (52 Mt), and oats (7.5 Mt). The USA produced the most corn (348.8 Mt). Table 1 presents a summary of data on the annual production of cereals by the largest food producers in the world, with information about the cultivation area and the use of pesticides [29].
Table 1.
| Region | Annual Production of Cereals * [million tonnes] | Crop Area * [million ha] |
Estimated Pesticide Usage [kg/ha] | Estimated Fungicide Usage [kg/ha] |
|---|---|---|---|---|
| World | 2166 | 482.5 | 117.9 | 48.4 |
| EU (27) | 254.3 | 47.5 | 38.8 (EU) | 19.4 (EU) |
| US | 398.6 | 47.9 | 4.3 | 0.3 |
| Brazil | 121.2 | 24.9 | 12.7 | 2.8 |
| China | 418.2 | 67.5 | 1.4 | 0.4 |
* Wheat, rye, barley, oats, corn.
3.3. Areas of Fungicide Action in Mold Cells
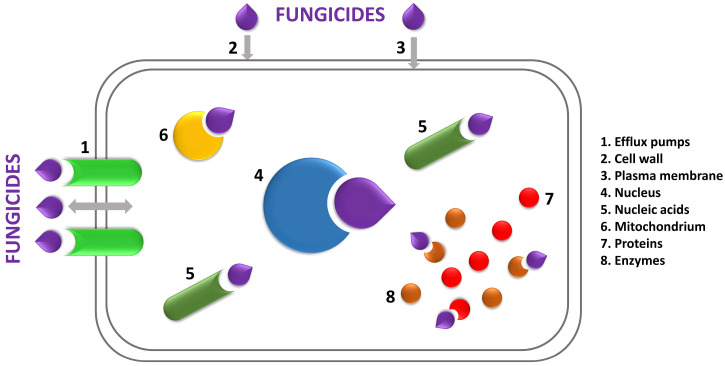
There are several main areas of action by antifungal preparations, including fungicides, in fungal cells (Figure 3). The outermost structure is the cell wall, which is the first place of contact between biocidal substances and the cell. The cell wall is responsible for the osmotic balance in the cell and controls the secretion and uptake of molecules into the cell. There are many enzymes and other substances in the cell wall, including color compounds (melanin). In most species of fungi, over 80% of the dry weight of the cell wall consists of polysaccharides, including chitin, glucan, and mannan. Another area in the cell affected by biocidal compounds is the cell membrane. Its main function is to control the permeability of various types of particles, including phospholipids, sphingolipids, sterols, and proteins. A characteristic sterol component in fungi is ergosterol, which is also found in mitochondrial membranes. Ergosterol plays an important role in the cell membrane because it influences its stiffness and thus its permeability. If the fungicide penetrates the cell wall and membrane, it reaches the interior of the cell, where it may interfere with DNA and protein synthesis or affect respiration. Mitosis and cell division may also be the targets of fungicides [1,32,33,34].
Figure 3.
Fungicide mechanisms of action in mold cells.
Information on the mechanisms and areas of action of individual groups of fungicide compounds can be found on the websites of the Fungicide Resistance Action Committee (FRAC). This committee confers on fungicides a special FRAC code, which allows them to be properly grouped. The classification applies to the biochemical mode of action in cell plant pathogens. Active substances can be targeted at a specific structure or enzyme, inhibiting a single metabolic process, or have a multidirectional range of action. The alternating use of different classes of protection agents with different mechanisms of action can prevent the development of fungal resistance [35]. It should be noted that the division of fungicides into FRAC groups does not always coincide with the division taking into account the chemical structure of the compounds. An example is benzimidazoles—a chemical group, but in the FRAC system they belong to Group 1 (MBC—mitosis inhibitors) along with other chemical compounds such as thiophanates [35].
3.4. Fungicide Groups According to Their Mode of Action (MOA) in the Biosynthetic Pathways of Plant Pathogens
An attractive target for antifungal agents is chitin, which is an integral component of the cell wall. The key enzyme catalyzing chitin biosynthesis is chitin synthase. An example of a fungicide that inhibits the action of this enzyme is polyoxin, which belongs to the 19 FRAC group [36,37]. The cell wall also contains melanin, a multifunctional pigment that allows fungi to survive in unfavorable conditions. Melanin not only protects the cell against environmental stress and UV light but also plays an important role in pathogenesis. Several enzymes are needed for the synthesis of melanin, such as reductase, dehydratase, and polyketide synthase. According to the divisions introduced by FRAC, we distinguish several groups acting on the biosynthesis of this pigment:
MBI-R (Melanin Biosynthesis Inhibitors—Reductase; 16.1 FRAC group) hydroxynaphthalene reductase inhibitors;
MBI-D (Melanin Biosynthesis Inhibitors—Dehydratase; 16.2 FRAC group) inhibiting scytalone dehydratase;
MBI-P (Melanin Biosynthesis Inhibitors—Polyketide synthase; 16.3 FRAC group) interfering with the activity of polyketide synthase.
All three types of fungicides can inhibit the production of melanin in fungal cells and, consequently, make them susceptible to unfavorable environmental conditions [38,39,40,41,42].
Another area of action of fungicides is the cell membrane, which consists largely of sterols and lipids. Lipid molecules are responsible for many biological functions in the fungal cell. They are an important reservoir of energy and are also part of the basic skeleton of the cell membrane. Specific proteins or enzymes needed for lipid metabolism therefore become the target of fungicides. The fungicide AH (Aromatic Hydrocarbons; 14 FRAC group) etridiazole hydrolyzes phospholipids of the cell membrane to produce lysophosphatides and free fatty acids, which in turn leads to the breakdown of membranes (lipid peroxidation). Another fungicide, AH dicloran, is a phototoxic compound that makes fungal membranes susceptible to sunlight, leading to the destruction of the linoleic acid structure. Dithiolates and phosphorothiolates (FRAC group 6) interfere with the biosynthesis of phospholipids by inhibiting the action of methyltransferases that catalyze methylation reactions. Carbamates (FRAC group 28) disrupt the permeability of the cell membrane, while plant extracts (FRAC group 46), such as tea tree extract (Melaleuca Alternifolia), cause disruption of the cell membrane. Polyene macrolides (FRAC group 48) bind ergosterol in the fungal cell membrane, which leads to the formation of hydrophilic channels. They change the permeability of the membrane, causing the outflow of intracellular contents. OSBPI (Oxysterol binding protein homolog inhibition; 49 FRAC group) fungicides inhibit the homolog of oxysterol binding protein (OSBP), which participates in the movement of lipid molecules between membranes. Inhibition of OSBP may also interfere with the maintenance of cell membranes and the formation of complex lipids necessary for cell survival [35,43,44].
A large group of fungicides are sterol biosynthesis inhibitors (SBI), which are capable of inhibiting various enzymes in the sterol biosynthesis pathway. Four classes of fungicides affecting the biosynthesis of sterols in the membrane can be distinguished: 1 SBI (DMI fungicides, demethylation inhibitors; 3 FRAC group), 2 SBI (amines, morpholines; 5 FRAC group), 3 SBI (KRI fungicides, ketoreductase inhibitors; 17 group FRAC), and 4 (18 FRAC group) [35]. DMI fungicides inhibit lanosterol 14α-demethylase, which is cytochrome P450 dependent. Morpholines lead to the inhibition of sterol Δ8, Δ7-isomerase, and sterol Δ14 reductase. Ketoreductase inhibitors inhibit 3-keto reductase and C4 demethylation. However, the 4th class of SBI fungicides, including e.g., naftifine and pyributicarb, is capable of inhibiting squalene epoxidase. Inhibition of even one of the enzymes necessary for sterol synthesis in the cell membrane leads to cell dysfunction and lysis [45,46].
An important structure in eukaryotic cells is the cytoskeleton, thanks to which the organelles do not float freely in the cytosol, but occupy certain places assigned to them. The polymers of the cytoskeleton are microtubules, which play an essential role in the cellular functions of fungi, e.g., cell division. They are therefore an important site for the action of biocidal compounds. An example is fungicides from FRAC group—MBC (Methyl Benzimidazole Carbamates) bind to the β-tubulin subunit in microtubules. This action inhibits the formation and proliferation of microtubules. By inhibiting mitosis, MBCs prevent the proper course of the fungal cell division process, resulting in its death. Microtubule polymerization inhibitors also include N-phenylcarbamates (FRAC group 10) and benzamide and thiazole carboxamide (FRAC group 22).
It is known that mycelium growth takes place in the top (apical) part of the hyphae. The stability of the membrane during elongation, especially at the hyphal tips, is determined by spectrin-like proteins located in this area. Their delocalization, caused by e.g., benzamides (FRAC group 43), results in inhibition of fungal growth. Other fungicides that affect the proper functioning of the cell are cyanoacrylates (FRAC group 47) and arylphenyl ketones (FRAC group 50). These probably interfere with the pathway that is responsible for regulating the actin cytoskeleton. They may also disturb the maintenance of cell polarity and hyphal morphogenesis, as well as inhibit the growth of polarized hyphae [47,48,49,50].
Fungi are aerobic organisms, and an important energy generator necessary for respiration are mitochondria. Mitochondria are the main regulator of the cellular metabolism of fungi and are the place where many physiological functions of the cell are coordinated. Hence, a FRAC group 39 of fungicides was created that inhibits the respiration of fungi. It consists of substances such as quinazoline, pyrimidines, and pyrazole-MET1, which act by influencing the complex I system of fungal mitochondria, inhibiting NADH oxidoreductases. SDHI fungicides (succinate dehydrogenase inhibitors; FRAC group 7) are complex II inhibitors that lead to dysfunction of the succinate dehydrogenase (SDH) enzyme in the mitochondrial electron transport chain and the Krebs cycle.
FRAC groups 11, 21, and 45 fungicides block electron transfer in mitochondrial complex III in cytochrome bc1. The first group of Quinone outside inhibitors (QoI) is capable of binding to cytochrome bc1 in the external quinoloxidizing pocket. Quinone inside inhibitors (QiI) bind in the internal quinone-reducing pocket. Quinone outside inhibitor stigmatellin binding type (QoSI) binds on the “outside” of the quinone in the stigmatellin binding subsite.
Fungicides belonging to the 29 FRAC group have an uncoupling effect on oxidative phosphorylation, which inhibits ATP production and interrupts the cellular metabolism of fungi. Organotin compounds (30 FRAC group) are inhibitors of oxidative phosphorylation and inhibit the action of ATP synthase. Thiophenecarboxamides (38 FRAC group) inhibit ATP transport [51,52].
The main building blocks of fungi are proteins consisting of amino acids. These proteins catalyze many biochemical reactions and participate in the formation of the cytoskeleton. Therefore, like sterols, proteins are an important cell component that can be targeted by the mechanisms of action of fungicides. According to the division into individual FRAC groups, we distinguish the following fungicides acting on proteins and amino acids:
AP (Anilino-Pyrimidines; FRAC group 9), which is capable of secreting hydrolases and inhibiting methionine biosynthesis;
enopyranuronic acid antibiotic (FRAC group 23), the active ingredient of which is blasticidin-S, which is capable of inhibiting protein biosynthesis by affecting ribosomal peptidyl transfer;
kasugamycin, the active ingredient of hexopyranosyl antibiotic (24 FRAC group, which inhibits translation initiation and thus inhibits protein synthesis;
glucopyranosyl antibiotics (FRAC group 25), which inhibit the initiation stage;
tetracycline antibiotics (FRAC group 41), which are elongation inhibitors in protein synthesis and act on ribosomes [35,53].
Another important process that takes place in fungal cells is signal transduction—i.e., a number of biochemical processes (signals) originating from outside the cell or from its interior, leading to changes in life processes in the cell. Signal transduction in the cell occurs at the level of the cell membrane and is related to the functions of certain proteins. Abnormalities in signal transmission may result in cell dysfunction and, consequently, lead to cell death. An example are phenylpyrroles (FRAC group 12), which interfere with the osmoregulatory signal transmission pathway, interfering with the os-2 MAP kinase pathway. In germinating spores, they stimulate the accumulation of glycerol, which leads to cell swelling and, consequently, its rupture. Dicarboximides (FRAC group 2) also act on the osmotic signal transduction pathway and interfere with the os-1 MAP kinase pathway. As a result of cutting off signal transmission, they can inhibit the synthesis of glycerol as well as the development of mycelial hyphae. Aza-naphthalenes (FRAC group 13) also influence signal transduction, but their mechanism of action is not yet known [35,44,54].
The last group of fungicides categorized according to their mechanism of action against plant pathogens are fungicides that affect the synthesis of nucleic acids. PA fungicides (PhenylAmides; FRAC group 4) interfere with the synthesis of nucleic acids by inhibiting the activity of the RNA polymerase I system. Hydroxy-(2-amino-)pyrimidines (FRAC group 8) are responsible for inhibiting the action of the adenosine deamination hydrolyzing enzyme. This results in disruption of inosine production and, consequently, inhibition of nucleic acid synthesis. Carboxylic acids (FRAC group 31) interfere with the action of topoisomerases necessary for the functioning of the cell. They are used as inhibitors of type II DNA topoisomerase directed at gyrase. In turn, heteroaromatic fungicides (FRAC group 32) interfere with DNA/RNA synthesis in the fungal cell [55,56].
It can be concluded that fungicides are a very wide group of compounds. They can influence plant pathogens in various ways, acting on many areas, processes, and individual chemical compounds in their cells to prevent fungal diseases from damaging and destroying plants.
3.5. Fungicides in Crop Protection
Fungal diseases present one of the main threats to crops, so the use of fungicides is often necessary to secure global food supplies. Due to their ease of use, effectiveness in combating fungal plant diseases, and relatively low cost compared to possible losses, the use of fungicides has become very common in agriculture [1,57]. Fungicides can be divided according to several criteria:
-
AStage of application:
- Preventive: These fungicides protect plants by preemptively guarding against pathogenic attacks;
- Interventional: Applied immediately after the onset of infection, these fungicides aim to halt further proliferation of the pathogen;
- Destructive: Designed to eradicate spores and mycelium, thereby curbing the spread of the disease.
-
BEffect of active substances of plants:
- Contact: These fungicides act on the plant’s surface, inhibiting spore germination and other vital processes;
- Translaminar: This type of fungicide penetrates and moves within the leaf tissue, providing protection throughout the leaf structure;
- Systemic: These fungicides penetrate plant tissues and distribute within them, creating a barrier against infection and fungal growth;
- Local: These fungicides exert their effects by penetrating only a few cell layers at the site of application
-
CMethods/places of application:
- Seed dressing: Application of finely ground solid particles dusted onto the seeds surface;
- Foliar fungicides: Sprayed onto the foliage of plants at different growth stages;
- Root/soil application: Soil drenching or injection into the soil underneath it near the roots.
-
D
Type of active substance used in fungicides.
-
E
Mechanisms of action of active substances on fungal cells.
Fungicides for agricultural crops can be used for both preventive and destructive purposes. Examples of the preventive use of fungicides include seed treatment, soil drenching, and spraying during the early developmental stages of plants. If preventative measures fail, destructive measures are used to manage the spores and mycelium. Most often, this involves spraying the plants with fungicide, as this allows for the fastest control of infectious foci [58].
There are many forms of fungicides currently available on the market, including dusts, gases, granules, and the most common form, which is liquid. Seed treatment fungicides can be applied in either wet or dry forms, depending on the type of seed. Powdering is a dry method used in seed protection, whereas wet methods include the usage of water dispersible powders, liquid solutions, and flowable solutions [7,24,59,60]. Fungicides can also be used during soil preparation and plant growth. When used on plants, the most efficient method is spraying, which can be performed as needed. Most often, foliar fungicides are applied on young plants in the preliminary stages of development to avoid infection. If infection is already present, the fungicide is used to eradicate fungal corruption and ensure that the pathogens will not be carried over into later developmental stages. The purpose of post-harvest use of fungicides is mostly to protect the harvested material against pathogens during storage [1]. Examples of fungicides used for seed treatment include captan, difenoconazole, and iprodione. Iprodione, as well as tebuconazole and pyraclostrobin, can also be used for foliar spraying. A more complete list of the most common fungicides for plant protection currently in use or recently phased out by large crop producers is given in Table 2. Of the 43 fungicides presented in the table, 27 are banned in the EU, 6 are banned in the USA, 11 are banned in China, and 9 are banned in Brazil. The groups with the most active substances include DMIs fungicides (demethylation inhibitors, 3 FRAC group)—11 substances; QoI (quinone outside inhibitors, 11 FRAC group)—5 substances; MBC (methyl benzimidazole carbamates, 1 FRAC group)—4 substances. The fungicide groups with the highest overall usage include dithiocarbamates (FRAC group M 03), DMIs (Demethylation Inhibitors, FRAC group 3), and QoIs (Quinone outside Inhibitors, FRAC group 11), especially strobilurins [57].
Table 2.
Examples of fungicides commonly used in cereal protection and their regulatory status in selected regions [35,61,62,63,64,65,66].
| Fungicide | Group Name (FRAC Group) |
Examples of Application Area |
Permitted Usage in Agriculture EU/USA/CHINA/BRAZIL | References |
|---|---|---|---|---|
| Morpholines | ||||
| Tridemorph | Amines (5) | Foliar spraying | −/+/+/− | [67] |
| Pyrimidines | ||||
| Cyprodinil | AP-fungicides (9) |
Foliar spraying, soil fumigant | +/+/+/+ | [68,69] |
| Carboxylic Acid Esters | ||||
| Ethyl formate | Carboxylic Acid Esters (n.a.) | Grain fumigant | −/?/?/? | [70] |
| Phthalonitriles | ||||
| Chlorothalonil | Chloronitriles (M 05) |
Foliar spraying | −/+/+/+ | [71] |
| Dicarboximides | ||||
| Iprodione | Dicarboximides (2) | Seed treatment, foliar spraying | −/+/+/+ | [72,73] |
| Dithiocarbamates | ||||
| Mancozeb | Dithiocarbamates (M 03) |
Seed treatment | −/+/+/+ | [74,75] |
| Metam-sodium | Soil fumigation | +/+/+/+ | [76] | |
| Thiram | Foliar spraying | −/+/+/+ | [77] | |
| Imidazoles, Triazoles | ||||
| Bitertanol | DMIs (3) |
Foliar spraying, seed treatment | −/+/+/− | [78] |
| Cyproconazole | Foliar spraying | −/+/−/+ | [79] | |
| Difenoconazole | Seed treatment | +/+/+/+ | [80,81] | |
| Imazalil | Seed treatment | +/+/+/+ | [82] | |
| Myclobutanil | Seed treatment, foliar spraying | −/+/+/+ | [83] | |
| Prochloraz | Seed treatment, foliar spraying | −/+/+/− | [84] | |
| Propiconazole | Seed treatment, foliar spraying | −/+/+/+ | [85] | |
| Tebuconazole | Foliar spraying | +/+/+/+ | [86,87] | |
| Triadimefon | Seed treatment | −/+/+/+ | [88] | |
| Triadimenol | Foliar spraying | −/+/+/+ | [89] | |
| Triticonazole | Seed treatment | +/+/+/+ | [90,91] | |
| Epoxides | ||||
| Ethylene oxide | Cyclic ethers (n.a.) |
Grain fumigant | −/−/−/− | [92] |
| 1,2,4-thiadiazoles | ||||
| Etridiazole | Heteroaromatics (32) | Seed treatment | −/+/−/+ | [93] |
| Inorganic compounds | ||||
| Aluminum phosphide | Aluminum, Copper salts etc. (NC) |
Grain fumigant | +/+/−/? | [94,95,96] |
| Copper oxychloride | Foliar spraying, seed treatment | +/+/+/+ | [97] | |
| Copper sulfate | Seed treatment | +/+/−/+ | [98] | |
| Benzimidazoles | ||||
| Benomyl | MBCs (1) |
Seed treatment, foliar spraying | −/−/+/− | [99,100] |
| Carbendazim | Foliar spraying, Seed treatment | −/+/+/+ | [101] | |
| Thiabendazole | Seed treatment | +/+/+/+ | [102] | |
| Thiophanates | ||||
| Thiophanate-methyl | MBCs (1) | Seed treatment | −/+/+/+ | [103] |
| Halogenated aliphatics | ||||
| Ethylene dibromide | Organobromine compounds (n.a.) |
Grain fumigant | −/−/−/− | [104] |
| Methyl bromide | Soil fumigation | −/−/−/− | [105] | |
| Chloropicrin | Organochlorine compounds (n.a.) |
Soil fumigant, grain fumigant | −/+/−/? | [106,107] |
| 1,3-dichloropropene | Soil fumigant | −/+/+/+ | [108,109] | |
| Ethylene dichloride | Grain fumigant | −/−/−/− | [110] | |
| Acylalanines | ||||
| Metalaxyl | Phenylamides (4) |
Seed treatment | +/+/−/+ | [111] |
| Phthalimides | ||||
| Captafol | Phthalimides (M 04) |
Seed treatment | −/−/−/− | [112,113] |
| Captan | Soil fumigant, seed treatment | +/+/+/+ | [114,115] | |
| Strobilurins | ||||
| Azoxystrobin | QoIs (11) |
Foliar spraying | +/+/+/+ | [99,116,117] |
| Trifloxystrobin | Foliar spraying | +/+/+/+ | [117] | |
| Kresoxim methyl | Foliar spraying | +/+/+/+ | [118] | |
| Pyraclostrobin | Foliar spraying | −/+/+/+ | [119] | |
| Oxazoles | ||||
| Famoxadone | QoIs (11) | Foliar spraying | +/+/+/+ | [120] |
| Oxathiins | ||||
| Carboxin | SDHIs (7) |
Seed treatment | −/+/+/+ | [121] |
| Oxycarboxin | Seed treatment, foliar spraying | −/+/+/+ | [122] | |
(+) Permitted, (−) Not permitted; (?) No data, n.a.—information unavailable.
Until recently, there was a large range of plant protection products available. However, due to more restrictive legal regulations, such as Regulation EC 1107/2009 [63] and Directive 2009/128/EC [123], many products have already been withdrawn or will be withdrawn soon. Substances withdrawn from circulation in the EU in 2020–2022 included frequently used fungicides such as benalaxyl, epoxiconazole, thiophanate methyl, triflumizole, carboxin, cyproconazole, diethofencarb, fenbuconazole, fluquinconazole, flutriafol, mancozeb, prochloraz, and triazoxide. In 2023, four fungicides were banned, namely benthiavalicarb, dimoxystrobin, ipconazole, and metiram. Additionally, 34 important fungicides for crop protection were planned for reassessment (and eventual withdrawal), including fluazinam, pyraclostrobin, cyprodinil, fosetyl, metconazole, boscalid, captan, fluoxastrobin, prothioconazole, tebuconazole, penconazole, and tetraconazole [124,125,126,127,128] (Reg. 2023/114; Reg. 2023/689; Reg. 2023/918; Reg. 2023/1446; Reg. 2023/2592). However, all 34 compounds have since received extensions of approval for use [25,63]. By 1 January 2024, of 1477 active substances used in pesticides registered in the EU database, 951 had been banned. In the literature, there are currently 490 fungicidal substances known. Not all of these 490 substances were previously registered in the EU. After comparison of the substances present in the literature and EU database, 175 of these are banned. Within those 175 fungicides, 78 were used in agriculture, with 71 potentially used for seed treatment. Notably, common grain protection fungicides including mancozeb, prochloraz, propiconazole, and thiophanate-methyl were among those withdrawn.
3.6. Synthetic Antifungals and the Environment
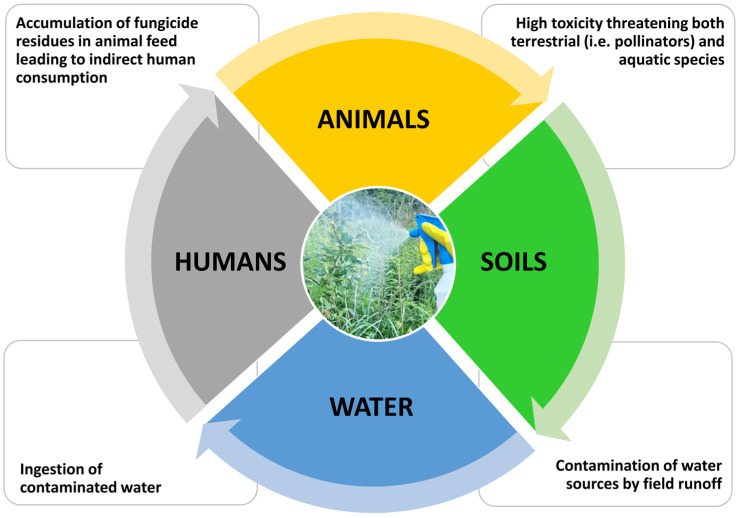
Many synthetic fungicides have been or will be withdrawn in the near future, primarily due to their toxicity to humans and the environment (Figure 4) [24]. An example of the tragic effects of pesticide (including fungicide) use is the decline in the number of birds in the United Kingdom during the period 1967–97. Data show that between 23% and 89% of birds died out in this period, depending on the species. In addition, these compounds accumulate in the soil and contaminate water as they run off fields, posing a threat to microbial diversity for long periods of time, depending on the ability of the substance used to break down over time [129,130,131,132]. In aquatic environments and agricultural fields, excessive concentrations of pesticides may cause a breakdown of the balance of microorganisms present in the environment, or even affect the crop itself [133]. Beneficial insect species such as pollinators may also suffer. For these reasons, a new generation of fungicides is sought that will not have a negative impact on soil structure and biocenosis. These new solutions should have low toxicity and aggregation capacity to ensure the safety of both humans and the environment.
Figure 4.
Fungicides and their impacts on various environmental zones.
Many factors affect the decomposition of pesticides used in agriculture, including access to light, temperature, and the microbiome. Depending on the combination of these factors, the half-life of pesticides can range from a week to a year [134]. The long-term degradation of synthetic fungicides causes serious environmental pollution. Furthermore, misuse and overuse of fungicides can be harmful for non-target species occurring in the environment and disturb its natural biodiversity. The degradation of synthetic fungicides can also create problems beyond the agricultural industry. An example is the development of cross-resistance in clinical strains, which through interactions with resistant environmental strains become resistant to azole drugs used in medicine. Aspergillus fumigatus found both in fields and in medical cases of aspergillosis is one of the best-known instances of this problem [135].
However, synthetic fungicides also have clear benefits. Fungicides are able to control many pathogenic fungi, thanks to which they can be used both for protective and destructive purposes in the event of infection. They thereby play an important role in preventing crop losses and increasing food safety, which is crucial in the context of an ever-growing population [136].
It seems clear that chemical agents will continue to be used, and new synthetic fungicides will be needed as current solutions become less effective over time. However, given the disadvantages of chemical plant protection products, it seems rational to combine the use of synthetic fungicides with natural biofungicides [7,137].
4. Biological Methods of Cereal Preservation
Biological control agents (BCAs) are a group of living organisms including microorganisms, insects, mites, nematodes, protozoa, and viruses, as well as botanical (plant extracts) and semiochemical (pheromones, kairomones) substances used to protect plants and seeds from pests and phytopathogenic diseases [138]. Synthetic agrochemicals are excluded from the group of BCAs. However, several substances occurring in nature that are equivalents of synthetic substances (semiochemicals) are permitted [139,140]. Although the term biological control has existed for over 100 years (introduced by Smith in 1919), the terminology in this area probably shows divergences depending on whether it is formulated by entomologists or biologists. In 2001, Eilenberg et al. [141] and later in 2017 Heimpel and Mills [142], proposed a harmonized definition for all biological control areas, but some inconsistencies still exist. The entry into force of Regulation (EC) No 1107/2009 on plant protection products (PPPs) [63] resulted in an increase in the use of BCAs in Europe. This regulation does not clearly define BCAs, and there is still a lack of a universally accepted definition of biocontrol. This may result in confusion and misuse among researchers and potential users regarding the safe use of biopreparations [143].
BCA-based seed treatment technology includes tools to improve seed quality, primarily reducing damage caused by pathogens and rot diseases. Both factors can drastically affect the germination process and therefore the final crop yield. However, seeds have developed their own passive physical (reinforced cell walls) and chemical (antimicrobial compounds) mechanisms, which are dependent on genetic and environmental factors, in response to attack by organisms and phytopathogens. These mechanisms are activated during seed germination, or in some cases during the rehydration cycle in the soil. In the dormant state (e.g., during storage), seeds are unable to activate defense mechanisms, suggesting that this is an appropriate period to install exogenous barriers as a preemptive strategy to protect grains [144,145]. The treatment of the seed surface with exogenous active substances (liquid or solid, forming a more or less continuous layer) using a binder or, in some cases, a filler that can act as a carrier has been proposed as a precise and cost-effective method [146,147]. Various types of seed treatment are used to improve the functional properties of seeds, including seed dressing, coating, soaking, and granulating, depending on the type and purpose of the seeds or the type of active ingredients selected for inoculation (whole microbial cells, natural active compounds, etc.) [147]. Seed coatings were first used in the 1930s for cereal grains, although their large-scale commercial use did not occur until 30 years later [148]. Nowadays, seed dressings are widely used in the crop and horticultural industries around the world to apply dyes, pesticides, soil adjuvants, germination and growth stimulants, substances that increase resistance to stress (e.g., salicylic acid, gibberellic acid, and abscisic acid), macronutrients and microelements, as well as biological control agents. The coatings with BCAs have been successfully applied to many different sizes, shapes, textures, and germination types of seeds, especially cereal grains [147]. Therefore, here, we will mainly focus on natural antifungal substances employed for protecting cereal grains.
4.1. Bacteria, Yeast, and Molds as Biocontrol Agents
The use of microorganisms as BCAs to protect against cereal seed-borne diseases is based mainly on their ability to synthesize a wide range of secondary metabolites (e.g., antibiotics, hydrogen cyanide) and lytic enzymes that can inhibit the growth of phytopathogens, as well as the activation of induced systemic resistance [149,150]. After the end of 2022, four new regulations (EU Regulation 2022/1438, 2022/1439, 2022/1440, 2022/1441) approved in the EU entered into force to simplify the approval process and authorization of biological plant protection products containing microorganisms. Currently, 71 strains of microorganisms are approved in the EU; another 26 strains are in the process of being approved [143].
The main microbial biocontrol agents with strong antifungal activity are Bacillus spp., which produce fungicidal and fungistatic metabolites. Endophytic strains of Bacillus subtilis, Bacillus velezensis, Bacillus inaquosorum, and Bacillus nakamurai inhibit the growth of Fusarium graminearum (Gibberella zeae) and F. poae on various cereal grains (barley, wheat) both in vitro, in greenhouse and under field conditions [151,152,153]. Pan et al. (2015) demonstrated the ability of B. subtilis and Bacillus megaterium species to inhibit the growth and spore germination of F. graminearum [154]. Bacillus vallismortis has been reported to have a wide spectrum of antifungal activity [155]. The filtrate and extract of B. vallismortis were found to inhibit the growth of several phytopathogens, including Fusarium graminearum, Alternaria alternata, Rhizoctonia solani, Cryphonectria parasitica, and Phytophthora capsici. The strong antagonistic activity of these species results from the secretion of antifungal lipopeptides, namely bacillomycins, iturins, surfactins, fengycins, and mycosubtilins [153]. Bacillus spp. is also known to synthesize hydrolytic enzymes such as amylase, cellulase, chitinase, lipase, phytase, and protease, important in biocontrol processes. These spore-forming bacteria are characterized by a high degree of adaptation to environmental stress conditions, which allows them to easily inhabit various niches. Due to the rapid colonization and utilization of nutrients (carbon, hydrogen, oxygen, nitrogen) and microelements (iron, manganese, copper, zinc, phosphorus) by Bacillus spp., they make the environment less favorable for the development of pathogens [156].
Other promising biocontrol agents for the protection of grains against Fusarium spp. include cell-free supernatants of lactic acid bacteria Lactiplantibacillus plantarum and Lacticaseibacillus rhamnosus [157,158].
The multidirectional antifungal activity of Streptomyces spp. has been demonstrated in many studies [150]. These bacteria are known to synthesize various secondary metabolites (e.g., 2-(chloromethyl)-2-cyclopropyloxirane, 2,4-ditert-butylphenol and 1-ethylthio-3-methyl-1,3-butadiene) that can inhibit the development of phytopathogens. They also (e.g., Streptomyces lydicus, S. mutabilis) produce enzyme—chitinase that can degrade the cell walls of various phytopathogenic fungi such as Fusarium spp. and Rhizoctonia solani [150,159]. In addition to bioactive substances and enzymes, many Streptomyces (e.g., Streptomyces platensis, S. noursei, S. fimicarius, S. albulus) are producers of volatile organic compounds (VOCs, e.g., anisole, alpha-copaene, caryophyllene, ethyl phenylacetate, methyl anthranilate, methyl salicylate phenylethyl alcohol, 4-methoxystyrene, 2-pentylfuran, and 4-ethylphenol) with antimicrobial activity against phytopathogenic species Botrytis cinerea, Rhizoctonia solani, Fusarium oxysporum, and Sclerotinia sclerotiorum [150,160].
Biocontrol ability is widespread among yeast species. Matić et al. (2014) found that seed coating with Pichia guilliermondii, Metschnikowia pulcherrima, and Sporidiobolus pararoseus inhibited growth of Fusarium fujikuroi [161]. Druvefors and Schnürer (2005) investigated the antagonistic activity of 57 different yeasts belonging to the Pichia (P. anomala, P. guillermondii, P. burtonii, P. farinose, P. membranifaciens) and Candida (C. silvicola, C. fennica, C. pelliculosa, C. silvicultrix) genera against Penicillium roqueforti, one of the most important spoilage molds in airtight-stored cereal grains [162]. Clonostachys rosea (formerly named Gliocladium roseum) also shows promising biocontrol activity. To date, its antifungal activity has been documented against 11 mold genera, including Fusarium, Alternaria, and Botrytis (Table 3), which are associated with the secretion of the cell wall-degrading enzymes chitinase, glucanase, and protease [163]. Jensen et al. (2000) used both stored (dried) and fresh conidia of Clonostachys rosea to treat cereal grains infected with Fusarium culmorum in field conditions. They achieved more than 80% disease control with both types of conidia [164].
Table 3.
Microorganisms as biocontrol agents.
| Microorganisms | Pathogens | Active Compounds |
References |
|---|---|---|---|
| Bacteria | |||
| Bacillus subtilis | Fusarium graminearum | surfactin, iturin, fengycin lipopeptides | [151,152,154] |
| Bacillus megaterium | Fusarium graminearum | not studied | [154] |
| Bacillus vallismortis |
Fusarium graminearum
Alternaria alternata Rhizoctonia solani Cryphonectria parasitica Phytophthora capsici |
bacillomycin D | [155] |
| Bacillus amyloliquefaciens |
F. graminearum Fusarium spp. |
iturin lipopeptide | [157,165] |
| Paenibacillus polymyxa | F. graminearum | not studied | [166] |
| Lacticaseibacillus rhamnosus | F. graminearum | not studied | [158] |
| Lactiplantibacillus plantarum |
F. graminearum Fusarium spp. |
not studied | [157,158] |
| Pseudomonas fluorescens | F. graminearum | not studied | [167] |
| Pseudomonas chlororaphis |
Drechslera graminea
D. teres D. avenae Ustilago avenae U. hordei Tilletia caries |
[168] | |
| Streptomyces spp. |
F. graminearum
Rhizoctonia solani |
secondary metabolites chitinases volatile organic compounds |
[150,167,168,169] |
| Azotobacter nigricans |
F. sporotrichioides
F. graminearum F. poae F. crookwellense F. equiseti F. sambucinum F. culmorum |
not studied | [170] |
| Yeasts | |||
| Sporidiobolus pararoseus | Fusarium fujikuroi | not studied | [161] |
| Pichia guilliermondii |
Fusarium fujikuroi
Penicillium roqueforti |
not studied | [161,162] |
| Metschnikowia pulcherrima | Fusarium fujikuroi | not studied | [161] |
| Cryptococcus flavescens | F. graminearum | not studied | [171] |
| Pichia anomala | Penicillium roqueforti | not studied | [162] |
| Pichia burtonii | |||
| Pichia farinosa | |||
| Pichia membranifaciens | |||
| Candida silvicola | |||
| Candida fennica | |||
| Candida pelliculosa | |||
| Candida silvicultrix | |||
| Molds | |||
| T. asperellum |
F. graminearum
Pseudomonas syringae |
not studied induced resistance |
[172] [173] |
| T. citrinoviride | F. graminearum | not studied | [158] |
| T. harzianum |
F. verticillioides
F. graminearum F. oxysporum B. cinerea |
secretion of chitinase, competition for space | [171,172,173,174] |
| T. brevicrasum | Rizoctonia solani | mycoparasitism | [173] |
| Clonostachys rosea |
F. culmorum F. graminearum F. verticillioides F. crookwellense Alternaria dauci A. radicina Botrytis cinerea B. aclada Bipolaris sorokiniana Drechslera teres Helminthosporium solani Moniliophthora roreri, Phytophthora palmivora Rhizoctonia solani Rhynchosporium communea Sclerotinia sclerotiorum |
secretion cell-wall-degrading enzymes | [163,164] |
A particularly promising strain for reducing the population of fungal pathogens, especially on cereal grains, is Trichoderma spp. [175]. As stated by Benitez et al. (2004), 90% of fungal strains used to control plant diseases belong to the genus Trichoderma. The most known and described BCA species are T. viride, T. virens, T. harzanium, T. hamatum, T. longibrachiatum, T. koningii, T. lixii, T. polysporum, and T. asperellum [176,177,178]. The success of Trichoderma spp. as a BCA is related to its multidirectional and comprehensive mechanisms. On the one hand, Trichoderma inhibits the development of phytopathogens such as Alternaria brassicicola, Armillaria mellea, Fusarium equiseti, F. guttiforme, F. graminearum, F. oxysporum, Phytophthora palmivora, Rhizoctonia solani, Sclerotium rolfsii, and Sclerotinia sclerotiorum [178,179]; on the other hand, they can stimulate growth and plant defense mechanisms. The wide spectrum of antagonistic activity of Trichoderma spp. is related to a combination of mycoparasitism, antibiosis, secretion of enzymes, and competition for space and nutrients [175,176,177,178,179,180]. It is considered as a necrotrophic mycoparasite and obtains nutrients by penetrating the pathogen’s cell wall without causing host cell death. Moreover, these fungi secrete cellular enzymes including chitinase, protease, glucanase, cellulase, xylanase, pectinase, lipase, and amylase, leading to the penetration and degradation of cell walls and causing plasmolysis [173,175]. Over 90 metabolites (low-molecular-weight volatile or non-volatile compounds) have also been detected in Trichoderma spp. (e.g., trichorzianin TA, trichorzianin TB, trichodermin, tricholin, cyclonerodiol, pachybasin, 6-pentyl-2H-pyran-2-one) with antifungal activity [181]. Xue et al. (2017) showed the strong antifungal activity of six strains of Trichoderma spp. (T. asperellum, T. citrinoviride, T. harzianum) against F. graminearum in wheat [172]. The same authors also showed that treating wheat seeds with spores of the tested mold species (107 spores/mL) reduced wheat root rot by >50%. Ferrigo et al. (2020) observed a 36% reduction in the occurrence of F. verticillioides and F. graminearum on grains coated with Trichoderma harzanium spores (105 spores/mL) [174].
In addition to inhibiting/limiting the growth of phytopathogens, microorganisms applied to cereal grains may perform another important function: detoxifying mycotoxins produced by fungi contaminating the grains. This is extremely important from the point of view of the dangers posed by the presence of aflatoxin B1 (AFB1), ochratoxins (OTA), fumonisins (FUM), deoxynivalenol (DON), zearalenone (ZEN), and T2/HT-2 toxins (T2). The most frequently detected mycotoxins in grains include OTA (wheat, barley, maize), ZEN (wheat, maize), DON (wheat, barley, maize), AFB1 (maize), and T2 (wheat, rye, oat, maize) [182]. According to research conducted by BIOMIN on cereals and cereal products, the most widespread mycotoxins in the world are DON (66%), FUMs (56%), and ZEN (53%) [183].
The ability to degrade mycotoxins is mainly demonstrated by molds, followed by bacteria and, less frequently, yeasts. Decontamination strategies to reduce mycotoxins are based on biodegradation, bioadsorption, or inhibition of mycotoxin production [184]. Biological control of mycotoxins offers an excellent alternative to chemical and physical methods to protect food and feed and is currently of great interest to researchers and industry. However, biodegradation may result in the formation of more toxic compounds. Therefore, it is necessary to analyze the toxicity of the resulting compounds [185].
According to the literature, nontoxigenic fungi including Aspergillus spp., Clonostachys spp., Penicillium spp., Rhizpous spp., and Trichoderma spp. are active agents reducing mycotoxins. Most studies have been conducted in vitro or in a simple system reflecting field conditions, with few in vivo studies directly on cereal grains. The literature data indicate that the most effective genus of fungi is Trichoderma. Błaszczyk et al. (2017) investigated 24 fungal strains of Trichoderma spp. (including T. atroviride, T. cremeum, T. hamatum, T. harzianum, T. longipile, T. viride, and T. viridescens) for inhibition of growth and mycotoxin biosynthesis by Fusarium spp. [186]. Trichoderma atroviride was found to have the ability to reduce not only DON and ZEN (69–100%), but also nivalenol, beauvericin, and moniliformin. The mycotoxin reduction capacity of Trichoderma atroviride and Trichoderma harzianum has also been the subject of research by Tian et al. (2018) [187]. These authors suggest that the mechanism of ZEN reduction by Trichoderma spp. is based on sulphation, which leads to the formation of zearalenone sulphate zearalenol sulphate. Trichoderma gamsii has been found to be an effective suppressor (28–68% reduction) of fumonisin B1 and fumonisin B2 in maize seeds [188]. According to Dini et al. (2022), Trichoderma afroharzianum can produce various enzymes, including glucanases, chitinases, and cellulases active against phytopathogens, but also peroxidases able to degrade mycotoxins such as AFB1 and OTA [189].
Many bacteria have the ability to detoxify one or more types of mycotoxins. The degree of mycotoxin detoxification depends not only on the bacterial strain but also on the substrate, pH, and temperature. Lactic acid bacteria are able to remove mycotoxins by cell-surface binding [190]. Lactic acid bacteria capable of cell-binding mycotoxins mentioned in the literature include Lactobacillus acidophilus, Lacticaseibacillus rhamnosus, Lactiplantibacillus plantarum, Lactobacillus amylovorus, and Limosilactobacillus fermentum. The mycotoxin binding efficiency of the mentioned LAB ranges from 60% to even 98% [190,191,192]. Streptomyces spp. and Bacillus spp. can remove mycotoxins via biodegradation. Harkai et al. (2016) examined 124 strains of Streptomyces spp. and showed that Streptomyces rimnosus was effective at biodegradation of ZEN mycotoxin (88% degradation), while Streptomyces cacaoi totally degraded AFB1 mycotoxin [185]. Bacillus spp. also have the potential to degrade ZEN and AFB1. Tinyiro et al. (2011) and Farzaneh et al. (2012) analyzed strains of B. subtilis, B. natto, and B. velezensis both in liquid cultures and on the food matrix of pistachio nuts [193,194]. It has also been shown that cell-free supernatant of B. subtilis and B. amyloliquefaciens reduced mycotoxin synthesis by A. parasiticus, A. niger, and Penicillium spp., and remained stable in the temperature range from −20 °C to 100 °C [195]. Yang et al. (2017) studied the ability of Pseudomonas fluorescens to degrade AFB1, with peanut kernels used as a matrix [196]. Over 88% detoxification was achieved after 96 h.
Yeast can degrade mycotoxins via biodegradation and bioadsorption. The ability to reduce mycotoxins (AFB1, OTA, and ZEN) by cell-binding has been demonstrated in Saccharomyces cerevisiae, S. uvarum, Kluyveromyces marxianus, and Candida utilis [197]. In the case of S. cerevisiae yeast, this mechanism is probably related to the presence of (1,3)-D-glucans in the cell wall [190].
Microbial biocontrol agents are considered a sustainable tool for the protection of crops, including cereals. Biofungicides can limit/inhibit the growth of phytopathogens, reduce the mycotoxins they produce, stimulate plant germination and growth, as well as enhance soil ecosystem function. They are biodegradable, friendly to non-target species, and do not cause phytopathogens to acquire resistance. Compared to synthetic fungicides, they have less adverse impact on the environment, with a shorter residual effect [198,199]. Nonetheless, questions remain about the safety of some forms of biological control. Goettel et al. (2001) identify allergenicity, toxicity, and pathogenicity as possible harmful effects of fungi used as biocontrol agents [200]. It is common knowledge that some species of fungi can cause allergies. There are no direct reports that fungi used as biocontrol agents are responsible for the production of common allergens, but such a risk exists with mass production and application. Both fungi and bacteria secrete a wide spectrum of compounds, many of which may be crucial in the biocontrol process, but which may also be toxic or pathogenic to plants, invertebrates, and vertebrates. Although most strains of biofungicides meet all biosafety requirements, there are potential risks associated with using the species Burkholderia cepacia, Pseudomonas putida, Pantoea agglomerans and Aureobasidium pullulans, which are considered opportunistic human pathogens [201]. It is worth noting that Trichoderma spp. is on the ever-growing list of fungal pathogens, although it rarely causes infections in humans. The most common pathogen of this genus causing infection is the species T. longibrachiatum. Therefore, its use in agriculture, should be limited. All microbial strains intended for use in agriculture should also be tested for possible pathogenicity, to minimize the risk of spreading diseases [202].
Another issue is the impact of biofungicides on the autochthonous microbiome in the environment. Most biofungicides are tested only under laboratory conditions. As noted by Bonaterra et al. (2012), before employing them in agriculture, it is necessary to monitor the behavior of microbial strains used as biocontrol factors in the ecosystem [201]. This includes their establishment, adaptation, survival, dispersal, genetic and phenotypic stability, and the risk of horizontal gene transfer (e.g., antibiotic resistance). It is important to ensure that, as a result of adaptation, biofungicides will not dominate the environment, disturbing the natural biodiversity of the microbial community. This seems to be the most difficult challenge for ensuring the safety of using biofungicides. Regulations in many countries already require environmental impact analysis of biopesticides as part of the registration and commercial development process [201].
Biocontrol agents are biomolecules with a short shelf life that require special formulations to guarantee appropriate effectiveness and stability [145]. One of the most significant challenges related to the use of BCAs in agriculture (which is less important in the case of grain protection) is their adaptation to environments highly exposed to pollution (including agrochemicals) and to changing environmental conditions (high/low temperatures, drought, heavy rainfall). The advantage of BCAs is that they are easily degraded but do not have lasting effects in the environment.
4.2. Antifungal Activities of Plant Extracts and Essential Oils on Grains
Plant extracts and essential oils (EOs) extracted from plants constitute an eco-friendly alternative to synthetic pesticides used on cereal grains, legumes, fruits, and vegetables. Prakash et al. (2012) found that EOs contain a mixture of various major and minor elements that are responsible for their biological activity [203]. This means that the chance of developing resistant fungal strains is lower than in the case of many synthetic fungicides used in agriculture. Some EOs have been shown to have not only strong fungicidal properties against molds, but also a remarkable ability to reduce mycotoxin synthesis. Taheri et al. (2023) list the mechanisms of antifungal action by EOs as follows: inhibition of cell wall formation, inhibition of cell division and development of the mitotic spindle, inhibition of nucleic acids and protein synthesis, inhibition of efflux pumps, disruption of cell membrane function, and mitochondrial dehydrogenase function [204,205]. The biological activity of EOs is attributed to the presence of main components such as bioactive terpenes, alkaloids, and phenolic aromatic molecules [206]. It appears that EOs with high levels of phenolic compounds have stronger antifungal properties.
Numerous studies have investigated the antifungal activity of various plant extracts and EOs from basil (Ocimum basilicum L.), rosemary (Rosmarinus officinalis L.), thyme (Thymus vulgaris L.), marjoram (Origanum majorana), ginger (Zingiber officinale), oregano (Origanum vulgare), cinnamon (Cinnamomum zeylanicum), palmarosa (Cymbopogon martinii), lemongrass (Cymbopogon citratus), clove (Eugenia caryophyllata), garlic (Allium sativum), lavender (Lavandula angustifolia Mill.), mint (Mentha piperita L.), sage (Salvia officinalis L.), tansy (Tanacetum vulgare L.), yarrow (Achillea millefolium L.), and wormwood (Artemisia absinthium L.) against, among others Aspergillus spp., Alternaria spp., Penicillium spp., and Fusarium spp. ([207,208,209] and references therein). However, the conclusions are still debated, especially since most of the research has been conducted in vitro under laboratory conditions and not directly on cereal grains during storage.
One of the many methods of applying essential oils to cereal grains is fumigation. Anžlovar et al. (2017) reported the antifungal potential of thyme essential oils against a group of endophytic fungi from wheat seeds, namely Alternaria alternata, Alternaria infectoria, Aspergillus flavus, Epicoccum nigrum, and Fusarium poae [210]. In the case of grains, fumigation has proven to be more beneficial than soaking because the EOs significantly inhibit fungal growth, while soaking the grains in EOs inhibits their germination. The use of EO from laurel (Laurus nobilis L.) and laurel components as antifungal agents for wheat grains has been investigated by Belasli et al. (2020) [211]. The laurel EO showed high effectiveness (51.5–76.7%) in protecting fumigated wheat grains against A. flavus contamination during storage for 6 months. Lee et al. (2021) evaluated the antifungal activity of lemongrass EO in the form of soaked sachets against Aspergillus flavus on wheat seeds stored for 30 days [212]. After the storage period, the lemongrass EO showed a high fungicidal effect, reducing fungal growth by nearly 100%.
Fumigation with essential oils has proven to be an effective process against phytopathogenic fungi not only on wheat grains but also other seeds. Ben Miri et al. (2023) studied the effect of fumigation with two EOs and combinations of these EOs on maize, barley, and rice grains [206]. Menthol, eugenol, and a mixture of both reduced mycelial growth and spore germination of A. ochraceus and A. niger stored in closed containers by over 50%. Roselló et al. (2015) demonstrated 90–100% effectiveness of cinnamon, clove, and oregano oils (at concentrations of 200 μg/mL) against fungal growth on rice grains stored at 28 °C for 30 days [213]. Essential oil of oregano showed potential to control the common fungal phytopathogens F. verticillioides and F. culmorum on rice seeds. Bocate et al. (2021) used fumigation with garlic EO on maize [214]. The growth of fungi Aspergillus parasiticus, Fusarium verticillioides, and Gibberella zeae was completely inhibited with concentrations of the EO ranging from 2 µg/L to 10 µg/L. On maize grains contaminated with Fusarium graminearum and Fusarium culmorum, Perczak et al. (2019) verified the effectiveness of EOs from oregano herb, cinnamon bark, palmarosa leaves, orange peel, verbena leaves and flowers, spearmint leaves, rosewood, and fennel seeds [215]. All of the tested oils reduced fungal growth without negatively affecting seed germination.
Sustainable agriculture practices that reduce reliance on synthetic chemicals are vital for ensuring food security while safeguarding the environment and human health. Currently, farmers rely heavily on synthetic fungicides to combat fungal diseases and prevent food losses. However, the widespread use of synthetic fungicides has led to the development of fungicide-resistant pathogens [216,217,218] and raised concerns about their impact on the environment and human health. BCAs have been proposed as an alternative, but their disease management capacity is often limited and dependent on unpredictable environmental factors. To achieve similar effectiveness as chemical fungicides, they must also be used in high doses, which often limits their application. Furthermore, the volatile and oxidizing nature of essential oils leads to high cost, which is also a significant limitation for large-scale use [207]. The integration of BCAs with fungicides is a promising approach to address these challenges, providing sustainable and effective solutions for managing plant diseases while mitigating the adverse effects of synthetic fungicides [189].
5. Integrated Control
Both biological control agents and plant extracts can constitute functional elements of an Integrated Pest Management System (IPM). Integrated Pest and Disease Management (IPDM) strategies have become increasingly essential in modern agriculture, driven by the need for sustainable practices that minimize the adverse effects of synthetic chemicals on both the environment and human health. While these integrated approaches hold great potential, further research is needed to optimize the timing and frequency of the application of BCAs and their compatibility with fungicides to maximize their impact on disease control and crop yield in various agricultural systems [219,220].
Integrating BCAs with fungicides can diversify antifungal treatments, reduce fungicide doses for disease management, and minimize residue on harvested crops. This approach also reduces the risk of pathogens developing resistance [221]. Numerous studies have explored the compatibility of various BCAs with fungicides across various crop systems. However, some experiments were carried out on fungicides that have now been withdrawn [222,223,224,225,226,227]. For instance, Sunkad et al. (2023) investigated the tolerance of Trichoderma species, including Trichoderma asperellum, against a range of fungicides [228]. The results revealed varying degrees of compatibility, with systemic fungicides such as azoxystrobin showing high compatibility with Trichoderma. The obtained fungicide concentrations inhibited all tested pathogenic strains without significantly inhibiting Trichoderma strains, the growth of which further enhanced the inhibitory effect of the fungicide-BCA combination. Similar studies have examined the compatibility of biocontrol yeasts Rhodosporidium kratochvilovae and Cryptococcus laurentii, and of bacteria Pseudomonas syringae with fungicides including boscalid, cyprodinil, fenhexamid, and thiabendazole. The results showed promise for controlling diseases including blue mold on apples [229,230].
Integrated approaches have also been developed and optimized for specific crop-disease systems. For example, in the case of tulsi wilt caused by Fusarium oxysporum, the integration of Trichoderma with the fungicides carbendazim and mancozeb has proven highly effective at reducing disease severity and enhancing plant growth [231]. This approach not only provides efficient disease management but also highlights the potential for eco-compatible agriculture. In the context of wheat diseases such as powdery mildew and Fusarium head blight (FHB), integrated approaches have been explored with BCAs including Rhodosporidium kratochvilovae, Cryptococcus laurentii, and Aureobasidium pullulans. These BCAs were combined with mineral salts, sulfur, and synthetic fungicides including azoxystrobin, tebuconazole, and tetraconazole. The results showed reduced disease severity, increased grain yield, and improved grain weight [232,233].
Integrated control does not end, however, with the combination of microorganisms and synthetic fungicides. Other approaches involve the combination of plant extracts/essential oils with fungicides, microorganisms, or organic compounds. In 2015, Fielding et al. (2015) examined the botryticidal effectiveness of South African medicinal plant extracts, both alone and with kresoxim-methyl fungicide [234]. Synergistic inhibitory effects were observed in vitro, and in vivo experiments on apples demonstrated synergistic and additive decay inhibition effects, suggesting the natural compounds in these plants enhance kresoxim-methyl efficacy [234]. Adandonon et al. (2006) investigated the efficacy of biocontrol agents, alone or combined with Moringa oleifera leaf extracts [235]. The results showed over 94% disease control in the greenhouse and 70% disease control in the field, with increases in yield. El-kazzaz et al. (2015) studied the efficacy of salicylic acid and plant extracts against rice kernel smut disease, recommending their inclusion in integrated pest management for combating Tilletia barclayana [236].
When describing integrated control, the topic of organic farming also cannot be ignored. Organic farming is a sustainable and environmentally friendly approach to agriculture that emphasizes the use of natural processes and inputs to grow crops. A core principle of organic farming is soil health [237]. Unlike conventional farming, which often relies on synthetic chemicals, organic farming focuses on maintaining ecological balance and enhancing biodiversity. Techniques such as crop rotation, green manure, cover cropping, and composting are adapted to maintain and improve soil quality and enhance its water retention capacity. To combat pests and diseases, organic farmers employ an integrated pest management (IPM) approach. It involves using a combination of biological, cultural, physical, and mechanical controls. In the case of fungal diseases, means such as crop rotation, breeding of resistant plant varieties, proper spacing, or natural fungicides are used. Crop rotation prevents build-up of pathogens in the soil by altering the crops that are susceptible to different diseases, while proper spacing reduces humidity by enhancing air circulation [238]. Natural fungicides include copper and sulfur-based products as well as biofungicides derived from beneficial microorganisms like Trichoderma or Bacillus species. Such practices allow for a system where chemical pesticides are not needed to control various dangers. However, while these methods are effective, it is not guaranteed that they will also work on large-scale farms, in the form they are employed in currently. Additionally, copper-containing fungicides might not be the perfect choice, as in his work Burandt et al. (2024) explains the potential risks of copper overuse from many different angles [239].
These studies collectively highlight the potential of plant extracts and organic compounds in combination with microorganisms or synthetic substances, as effective and environmentally friendly strategies for pest and disease management in agriculture. However, more work is needed to study the interactions of plant extracts with both fungicides and microorganisms exhibiting inhibitory capabilities against mold growth. Better understanding of these interactions would improve our ability to realistically assess the utility and applicative possibilities of integrated solutions in various agricultural areas.
Due to the lack of universal legislation, active substances may be allowed or prohibited in different parts of the world. This can create problems for comparison of experimental data. There is a need to keep the data as up to date as possible to ensure that future experiments will use substances that are permitted by the regional authorities, allowing for better data comparison between different microorganisms.
6. Summary
Synthetic (chemical) fungicides are currently the most common means for protecting plants and grains against phytopathogens. Farmers use them to protect their crops against the constant risk of infection with phytopathogens and, consequently, prevent economic losses. However, synthetic fungicides can also have a negative impact on the natural environment, including human and animal health. Moreover, when fungicides are overused, applied at sub-lethal doses, or used inappropriately, fungi can develop resistance to these chemicals. Concerns about their impact on human health, the environment, and the development of resistant pathogens have led to increased scrutiny and regulation. In response, many regions, including the European Union, have introduced restrictions on the use of synthetic fungicides.
Biological control agents appear to be a safe alternative to chemical fungicides for plant and seed protection. Biological control agents can help reduce the reliance on chemical fungicides. However, the complete or partial elimination of synthetic fungicide usage would create a gap that is difficult to fill in the short term. Furthermore, natural fungicides also have some disadvantages (Table 4). The choice between synthetic and natural fungicides for plant and seed treatments depends on many factors, including the type of crop, environmental conditions, and local regulations governing the use of plant protection products. It is important to strike a balance between producing efficient crops and protecting the environment and human health.
Table 4.
Summary of advantages and disadvantages of natural and synthetic fungicides.
| Antifungals | Advantages | Disadvantages |
|---|---|---|
| Natural |
|
|
| Synthetic |
|
|
In summary, the choice of preparations used to protect plants in agriculture is not always simple or straightforward. A promising approach seems to be the appropriate integration of natural and synthetic agents. Such integrated strategies can help to provide an effective solution to plant disease control problems while mitigating the adverse effects of synthetic fungicides alone. It is important to ensure that synthetic fungicides are used effectively so that they can play a part in more sustainable agriculture. To fully realize the potential of integrated approaches in responsible agricultural management, further investment and development in the following areas are necessary:
Registration procedures and regulatory guidelines;
Research tools that monitor and evaluate the risk of long-term use, in terms of maintaining the biodiversity of natural environments and, above all, the safety of people and animals.
With further development of technology, solutions applied directly to plants might soon be complemented by advanced approaches such as precision agriculture (or precision farming). This method utilizes remote sensing, yield monitoring, robotics, machine learning, and data analysis to boost efficiency and productivity in agriculture. Through data analysis and machine learning, the usage of fertilizers, pesticides, and water can be optimized, thereby reducing the overall costs of food production [240]. Additionally, collected data could become a valuable source of information for developing highly effective Integrated Pest Management (IPM) strategies [241]. Fungicides, which are crucial for controlling fungal diseases in crops, fit seamlessly into the framework of precision agriculture. The integration of fungicides into this advanced agricultural approach can lead to solutions such as the targeted application of fungicides (e.g., using the fungicide in the areas of the field that are most prone to infections) or applying the fungicide at optimal times to reduce the need for blanket applications, which could further reduce their use. The integration of fungicides into precision agriculture will likely enhance their effectiveness while reducing environmental and economic costs. By overcoming current hurdles such as high costs, technology integration, and education, precision agriculture, coupled with innovative fungicide developments, promises a more sustainable and productive future for farming [242,243].
Acknowledgments
This paper has been completed while the first author was the Doctoral Candidate in the Interdisciplinary Doctoral School at the Lodz University of Technology, Poland.
Author Contributions
Conceptualization, A.K.; methodology, T.S. and A.O.; software, A.O.; formal analysis, A.K.; investigation, T.S., A.K. and A.O.; data curation, A.K.; writing—original draft preparation, T.S., A.K. and A.O.; writing—review and editing, T.S., A.K. and A.O.; visualization, A.K. and A.O.; supervision, A.K.; funding acquisition, A.K. and A.O. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Baibakova E.V., Nefedjeva E.E., Suska-Malawska M., Wilk M., Sevriukova G.A., Zheltobriukhov V.F. Modern fungicides: Mechanisms of action, fungal resistance and phytotoxic effects. Annu. Res. Rev. Biol. 2019;32:1–16. doi: 10.9734/arrb/2019/v32i330083. [DOI] [Google Scholar]
- 2.Dias M. Phytotoxicity: An overview of the physiological responses of plants exposed to fungicides. J. Bot. 2012:135479. doi: 10.1155/2012/135479. [DOI] [Google Scholar]
- 3.Finch S., Samuel A., Lane G.P. Lockhart and Wiseman’s Crop Husbandry Including Grassland. 9th ed. Technology and Nutrition; Cambridge, UK: 2014. pp. 119–157. (Woodhead Publishing Series in Food Science). [Google Scholar]
- 4.Beck B.D., Matzen N., Madsen H.P., Kirkegaard S.S., Nielsen C.A.S., Nørholm S.R., Almskou-Dahlgaard A., Meyer A., Jørgensen L.N. Applied Crop Protection. Volume 226. DCA-Nationalt Center for Fødevarer og Jordbrug; Tjele, Denmark: 2024. Disease control in wheat; pp. 17–38. [Google Scholar]
- 5.Köycü N.D., Sukut F. Effect of the timing of fungicide application on yield and quality parameters of wheat infected with Fusarium crown rot disease. Black Sea J. Agric. 2024;7:113–120. doi: 10.47115/bsagriculture.1398334. [DOI] [Google Scholar]
- 6.Oerke E.C., Dehne H.W. Safeguarding production—Losses in major crops and the role of crop protection. Crop Prot. 2004;23:275–285. doi: 10.1016/j.cropro.2003.10.001. [DOI] [Google Scholar]
- 7.Thind T.S. Changing trends in discovery of new fungicides: A perspective. Indian Phytopathol. 2021;74:875–883. doi: 10.1007/s42360-021-00411-6. [DOI] [Google Scholar]
- 8.Russell P.E. A Century of Fungicide Evolution. J. Agric. Sci. 2005;143:11–25. doi: 10.1017/S0021859605004971. [DOI] [Google Scholar]
- 9.Lundgren K.D., Swensson A. A survey of results of investigations on some organic mercury compounds used as fungicides. Am. Ind. Hyg. Assoc. J. 1960;21:308–311. doi: 10.1080/00028896009343363. [DOI] [PubMed] [Google Scholar]
- 10.Qadri R., Azam M., Khan M.I., Yang Y., Ejaz S., Akram M.T., Khan M.A. Plant Disease Management Strategies for Sustainable Agriculture through Traditional and Modern Approaches. Volume 13. Springer; Cham, Switzerland: 2020. Conventional and modern technologies for the management of post-harvest diseases; pp. 137–172. [DOI] [Google Scholar]
- 11.Horsfal J.G. Fungi and fungicides: The story of a nonconformist. Annu. Rev. Phytopathol. 1975;13:1–14. doi: 10.1146/annurev.py.13.090175.000245. [DOI] [PubMed] [Google Scholar]
- 12.Klittich C.J. Milestones in fungicide discovery: Chemistry that changed agriculture. Plant Health Prog. 2008;9:31. doi: 10.1094/PHP-2008-0418-01-RV. [DOI] [Google Scholar]
- 13.A Short History of Fungicides. [(accessed on 16 December 2023)]. Available online: https://www.apsnet.org/edcenter/apsnetfeatures/Pages/Fungicides.aspx/
- 14.Krämer W., Schirmer U. Modern crop protection compounds. In: Krämer W., Schirmer U., editors. Crop Protection. Wiley-VCH; Weinheim, Germany: 2007. pp. 415–746. [Google Scholar]
- 15.Food Safety Commission of Japan Dichlobentiazox (pesticides) Food Saf. 2020;8:6–7. doi: 10.14252/foodsafetyfscj.D-20-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian Z., Li J., Zhang F., Xu L., Zhou F., Zhou L., Wang H., Liu R. Effects of mixtures containing physcion and several fungicides on the yield of wheat by seed coating and its potential mechanisms. Agriculture. 2024;14:237. doi: 10.3390/agriculture14020237. [DOI] [Google Scholar]
- 17.Leadbeater A. Recent developments and challenges in chemical disease control. Plant Prot. Sci. 2015;51:163–169. doi: 10.17221/83/2015-PPS. [DOI] [Google Scholar]
- 18.Mahapatra S., Chakraborty S., Debnath D., Roy C. Insights into wheat blast: Its epidemiology, recent advances and managements strategies. J. Crop Health. 2024;76:397–409. doi: 10.1007/s10343-023-00964-8. [DOI] [Google Scholar]
- 19.Fungicide Resistance Management; Fact Sheet EPP. [(accessed on 16 December 2023)]. Available online: https://shareok.org/bitstream/handle/11244/334679/oksa_EPP-7663_2009-04.pdf?sequence=1.
- 20.De Mio L.L.M., Peres N.A., Schnabel G., Isshi H. A special isssue on fungicide resistance and management strategies. Trop. Plant Pathol. 2024;49:1–4. doi: 10.1007/s40858-024-00648-2. [DOI] [Google Scholar]
- 21.Matelionienė N., Žvirdauskienė R., Kadžienė G., Zavtrikovienė E., Supronienė S. In Vitro sensitivity test of Fusarium species from weeds and non-gramineous plants to triazole fungicides. Pathogens. 2024;13:160. doi: 10.3390/pathogens13020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corkley I., Fraaije B., Hawkins N. Fungicide resistance management: Maximizing the effective life of plant protection products. Plant Pathol. 2022;71:150–169. doi: 10.1111/ppa.13467. [DOI] [Google Scholar]
- 23.Majeed A., Muhammad Z., Ullah Z., Ullah R., Ahmad H. Late blight of potato (Phytophthora infestans) I: Fungicides application and associated challenges. Turk. J. Agric. Food Sci. Technol. 2017;5:261–266. doi: 10.24925/turjaf.v5i3.261-266.1038. [DOI] [Google Scholar]
- 24.Lamichhane J.R., Dachbrodt-Saaydeh S., Kudsk P., Messéan A. Conventional pesticides in agriculture: Benefits versus risks. Plant Dis. 2016;100:10–24. doi: 10.1094/PDIS-05-15-0574-FE. [DOI] [PubMed] [Google Scholar]
- 25.Jess S., Kildea S., Moody A., Rennick G., Murchie A.K., Cooke L.R. European Union policy on pesticides: Implications for agriculture in Ireland. Pest Manag. Sci. 2014;70:1646–1654. doi: 10.1002/ps.3801. [DOI] [PubMed] [Google Scholar]
- 26.Suleiman R., Rosentrater K.A. Current maize production, postharvest losses and the risk of mycotoxins contamination in Tanzania; Proceedings of the ASABE International Meeting Presentation; New Orleans, LA, USA. 26–29 July 2015; [DOI] [Google Scholar]
- 27.Kumar D., Kalita P. Reducing postharvest losses during storage of grain crops to strengthen food security in developing countries. Foods. 2017;6:8. doi: 10.3390/foods6010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crop Protection Research and Reports. [(accessed on 16 April 2024)]. Available online: https://www.mordorintelligence.com/market-analysis/crop-protection.
- 29.Statistics Division Food and Agriculture Organization of the United Nations. [(accessed on 16 December 2023)]. Available online: https://www.fao.org/faostat/en/#data/RP.
- 30.Crops and Livestock Products. [(accessed on 16 December 2023)]. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize.
- 31.Crop Yields. [(accessed on 18 February 2024)]. Available online: https://ourworldindata.org/crop-yields#explore-data-on-crop-yields.
- 32.Denyer S.P. Mechanisms of action of biocides. Int. Biodeterior. 1990;26:89–100. doi: 10.1016/0265-3036(90)90050-H. [DOI] [Google Scholar]
- 33.Russell A.D. Similarities and differences in the responses of microorganisms to biocides. J. Antimicrob. Chemother. 2003;52:750–763. doi: 10.1093/jac/dkg422. [DOI] [PubMed] [Google Scholar]
- 34.Ghannoum M.A., Rice L.B. Antifungal agents: Mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 1999;12:501–517. doi: 10.1128/CMR.12.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.FRAC Code List 2024: Fungal Control Agents Sorted by Cross Resistance Pattern and Mode of Action (Including Coding for FRAC Groups on Product Labels) [(accessed on 4 June 2024)]. Available online: https://www.frac.info/docs/default-source/publications/frac-code-list/frac-code-list-2024.pdf?sfvrsn=52e14e9a_2.
- 36.Chen Q., Zhang J.W., Chen L.L., Yang J., Yang X.L., Ling Y., Yang Q. Design and synthesis of chitin synthase inhibitors as potent fungicides. Chin. Chem. Lett. 2017;28:1232–1237. doi: 10.1016/j.cclet.2017.03.030. [DOI] [Google Scholar]
- 37.Kurland C., Olsen G. Current opinion in microbiology: Editorial overview. Curr. Opin. Microbiol. 2002;5:497–498. doi: 10.1016/S1369-5274(02)00363-6. [DOI] [Google Scholar]
- 38.Banba S., Hamada T., Araki N., Ebihara K. Synthesis and activities of tolprocarb derivatives against Pyricularia oryzae: Relationships among the activities for polyketide synthase, melanin biosynthesis, and rice blast. J. Pestic. Sci. 2017;42:25–31. doi: 10.1584/jpestics.D16-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eisenman H.C., Casadevall A. Synthesis and assembly of fungal melanin. Appl. Microbiol. Biotechnol. 2012;93:931–940. doi: 10.1007/s00253-011-3777-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kimura N., Fukuchi A. Management of melanin biosynthesis dehydratase inhibitor (MBI-D)-resistance in Pyricularia oryzae using a non-MBI-D fungicidal application program for nursery boxes and a diclocymet and ferimzone mixture for field foliar applications. J. Pestic. Sci. 2018;43:287–292. doi: 10.1584/jpestics.D18-023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee J.K., Jung H.M., Kim S.Y. 1,8-Dihydroxynaphthalene (DHN)-Melanin Biosynthesis increase erythritol production in Torula corallina, and DHN-melanin inhibits erythrose reductase. Appl. Environ. Microbiol. 2003;69:3427–3434. doi: 10.1128/AEM.69.6.3427-3434.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Motoyama T., Yamaguchi I. Fungicides, Melanin Biosynthesis Inhibitors. In: Plimmer J.R., Gammon D.W., Ragsdale N.A., editors. Encyclopedia of Agrochemicals. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2003. [DOI] [Google Scholar]
- 43.Pan J., Hu C., Yu J.H. Lipid biosynthesis as an antifungal target. J. Fung. 2018;4:50. doi: 10.3390/jof4020050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang C., Hamel C., Vujanovic V., Gan Y. Fungicide: Modes of Action and Possible Impact on Nontarget Microorganisms. John Wiley & Sons; Hoboken, NJ, USA: 2011. 8p. [DOI] [Google Scholar]
- 45.Nes W.D. Biosynthesis of cholesterol and other sterols. Chem. Rev. 2011;111:6423–6451. doi: 10.1021/cr200021m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Umate P. Comparison of Genes encoding enzymes of sterol biosynthesis from plants to orthologs in yeast. Rice Res. 2016;4:160. doi: 10.4172/2375-4338.1000160. [DOI] [Google Scholar]
- 47.Opalski K.S., Tresch S., Kogel K.-H., Grossmann K., Köhle H., Hückelhoven R. Metrafenone: Studies on the mode of action of a novel cereal powdery mildew fungicide. Pest Manag. Sci. 2006;62:393–401. doi: 10.1002/ps.1176. [DOI] [PubMed] [Google Scholar]
- 48.Rogalska A., Miśkiewicz K., Marczak A. Inhibitors of microtubule polymerization- new natural compounds as potential anti-cancer drugs. Postepy Hig. Med. Dosw. 2015;69:571–585. doi: 10.5604/17322693.1151293. [DOI] [PubMed] [Google Scholar]
- 49.Vela-Corcía D., Romero D., De Vicente A., Pérez-García A. Analysis of β-tubulin-carbendazim interaction reveals that binding site for MBC fungicides does not include residues involved in fungicide resistance. Sci. Rep. 2018;8:7161. doi: 10.1038/s41598-018-25336-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wildman H.G., Lyr H., Russell P.E., Sisler H.D. Modern fungicides and antifungal compounds. Mycologia. 1997;89:820. doi: 10.2307/3761141. [DOI] [Google Scholar]
- 51.Calderone R., Li D., Traven A. System-level impact of mitochondria on fungal virulence: To metabolism and beyond. FEMS Yeast Res. 2015;15:fov027. doi: 10.1093/femsyr/fov027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leroux P., Gredt M., Leroch M., Walker A.S. Exploring mechanisms of resistance to respiratory inhibitors in field strains of Botrytis cinerea, the causal agent of gray mold. Appl. Environ. Microb. 2010;76:6615–6630. doi: 10.1128/AEM.00931-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ujváry I. Pest control agents from natural products. In: Krieger R., editor. Hayes’ Handbook of Pesticide Toxicology. 3rd ed. Elsevier; New York, NY, USA: 2010. pp. 119–229. [Google Scholar]
- 54.Zhang Y., Lamm R., Pillonel C., Lam S., Xu J.-R. Osmoregulation and fungicide resistance: The Neurospora crassa os-2 gene encodes a HOG1 mitogen-activated protein kinase homologue. Appl. Environ. Microbiol. 2002;68:532–538. doi: 10.1128/AEM.68.2.532-538.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jaswal S., Nehra B., Kumar S., Monga V. Recent advancements in the medicinal chemistry of bacterial type II topoisomerase inhibitors. Bioorg. Chem. 2020;104:104266. doi: 10.1016/j.bioorg.2020.104266. [DOI] [PubMed] [Google Scholar]
- 56.Ypema H. Fungicides, Hymexazol. In: Plimmer J.R., Gammon D.W., Ragsdale N.A., editors. Encyclopedia of Agrochemicals. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2003. [DOI] [Google Scholar]
- 57.Zubrod J.P., Bundschuh M., Arts G., Brühl C.A., Imfeld G., Knäbel A., Payraudeau S., Rasmussen J.J., Rohr J., Scharmüller A., et al. Fungicides: An overlooked pesticide class? Environ. Sci. Technol. 2019;53:3347–3365. doi: 10.1021/acs.est.8b04392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Poole N.F., Arnaudin M.E. The role of fungicides for effective disease management in cereal crops. Can. J. Plant Path. 2014;36:1–11. doi: 10.1080/07060661.2013.870230. [DOI] [Google Scholar]
- 59.Ayesha M., Suryanarayanan T., Nataraja K., Prasad S., Shaanker R. Seed treatment with systemic fungicides: Time for review. Front. Plant Sci. 2021;12:654512. doi: 10.3389/fpls.2021.654512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ahmed S., Kumar S. Seed coating with fungicides and various treatments for protection of crops: A review. Int. J. Agric. Environ. Sustain. 2020;2:6–13. [Google Scholar]
- 61.Pesticides. [(accessed on 18 February 2024)]. Available online: https://ourworldindata.org/pesticides.
- 62.PAN International Consolidated List of Banned Pesticides. [(accessed on 16 December 2023)]. Available online: https://pan-international.org/pan-international-consolidated-list-of-banned-pesticides/
- 63.Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 Concerning the Placing of Plant Protection Products on the Market and Repealing Council Directives 79/117/EEC and 91/414/EEC. European Union; Maastricht, The Netherlands: 2009. [(accessed on 4 August 2024)]. Available online: https://eur-lex.europa.eu/eli/reg/2009/1107/oj. [Google Scholar]
- 64.NPRO (NPIC, 2024. Product Research Online) 2024. [(accessed on 24 March 2024)]. Available online: http://npic.orst.edu/NPRO.
- 65.EPA United States Environmental Protection Agency Central Data Exchange. [(accessed on 24 March 2024)]; Available online: https://cdx.epa.gov.
- 66.Donley N. The USA lags behind other agricultural nations in banning harmful pesticides. Environ. Health. 2019;18:44. doi: 10.1186/s12940-019-0488-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gupta P.K. Toxicity of fungicides. In: Gupta R.C., editor. Veterinary Toxicology. 3rd ed. Academic Press; Cambridge, MA, USA: 2018. pp. 569–580. [DOI] [Google Scholar]
- 68.Shen C., Tang C., Zhu K., He C., Yang C., Zuo Z. Toxicity and ecological risk assessment for two AhR agonistic pesticides mepanipyrim and cyprodinil and their metabolites. Environ. Sci. Pollut. Res. 2023;30:58944–58955. doi: 10.1007/s11356-023-26735-6. [DOI] [PubMed] [Google Scholar]
- 69.Lv L., Li W., Li X., Wang D. Photodegradation and oxidation of fungicide cyprodinil by advanced oxidation processes (AOPs): Transformation products and toxicity assessment. J. Hazard. Mater. 2021;416:125933. doi: 10.1016/j.jhazmat.2021.125933. [DOI] [PubMed] [Google Scholar]
- 70.Bharathi V.S.K., Jayas D.S. Ethyl formate: A comprehensive review on its Function as a fumigant for stored products. J. Stored Prod. Res. 2024;106:102280. doi: 10.1016/j.jspr.2024.102280. [DOI] [Google Scholar]
- 71.Kiefer K., Bader T., Minas N., Salhi E., Janssen E.M.-L., von Gunten U., Hollender J. Chlorothalonil transformation products in drinking water resources: Widespread and challenging to abate. Water Res. 2020;183:116066. doi: 10.1016/j.watres.2020.116066. [DOI] [PubMed] [Google Scholar]
- 72.Carneiro L.S., Martínez L.C., Gonçalves W.G., Santana L.M., Serrão J.E. The fungicide iprodione affects midgut cells of non-target honey bee Apis mellifera workers. Ecotoxicol. Environ. Saf. 2020;189:109991. doi: 10.1016/j.ecoenv.2019.109991. [DOI] [PubMed] [Google Scholar]
- 73.Oiki S., Yaguchi T., Urayama S.-I., Hagiwara D. Wide distribution of resistance to the fungicides fludioxonil and iprodione in Penicillium species. PLoS ONE. 2022;17:e0262521. doi: 10.1371/journal.pone.0262521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dall’Agnol J.C., Ferri Pezzini M., Suarez Uribe N., Joveleviths D. Systemic effects of the pesticide mancozeb—A literature review. Eur. Rev. Med. Pharmacol. Sci. 2021;25:4113–4120. doi: 10.26355/eurrev_202106_26054. [DOI] [PubMed] [Google Scholar]
- 75.Quds R., Hashmi M.A., Iqbal Z., Mahmood R. Interaction of mancozeb with human hemoglobin: Spectroscopic, molecular docking and molecular dynamic simulation studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022;280:121503. doi: 10.1016/j.saa.2022.121503. [DOI] [PubMed] [Google Scholar]
- 76.Kaikai N.-E., Ba-M’hamed S., Ghanima A., Bennis M. Exposure to metam sodium-based pesticide impaired cognitive performances in adult mice: Involvement of oxidative damage and glial activation. Toxicol. Appl. Pharmacol. 2023;477:116677. doi: 10.1016/j.taap.2023.116677. [DOI] [PubMed] [Google Scholar]
- 77.Liu K., Li Y., Iqbal M., Tang Z., Zhang H. Thiram exposure in environment: A critical review on cytotoxicity. Chemosphere. 2022;295:133928. doi: 10.1016/j.chemosphere.2022.133928. [DOI] [PubMed] [Google Scholar]
- 78.Liu Y., Guo L., Xu X., Kuang H., Liu L., Xu C., Sun M. Immunochromatographic visualization detection platform for bitertanol in foods. Food Chem. 2024;444:138599. doi: 10.1016/j.foodchem.2024.138599. [DOI] [PubMed] [Google Scholar]
- 79.He R., Fan J., Chen R., Guo D., Zhao M., Zhang Z., Liang C., Chen M., Song H., Zhang W. Stereoselective in vitro metabolism of cyproconazole in rat liver microsomes and identification of major metabolites. Chemosphere. 2021;264:128495. doi: 10.1016/j.chemosphere.2020.128495. [DOI] [PubMed] [Google Scholar]
- 80.Wang X., Ni H., Xu W., Wu B., Xie T., Zhang C., Cheng J., Li Z., Tao L., Zhang Y. Difenoconazole induces oxidative DNA damage and mitochondria mediated apoptosis in SH-SY5Y cells. Chemosphere. 2021;283:131160. doi: 10.1016/j.chemosphere.2021.131160. [DOI] [PubMed] [Google Scholar]
- 81.Liu R., Li J., Zhang L., Feng T., Zhang Z., Zhang B. Fungicide Difenoconazole Induced Biochemical and Developmental Toxicity in Wheat (Triticum aestivum L.) Plants. 2021;10:2304. doi: 10.3390/plants10112304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Han L., Xu H., Wang Q., Liu X., Li X., Wang Y., Nie J., Liu M., Ju C., Yang C. Deciphering the degradation characteristics of the fungicides imazalil and penflufen and their effects on soil bacterial community composition, assembly, and functional profiles. J. Hazard. Mater. 2023;460:132379. doi: 10.1016/j.jhazmat.2023.132379. [DOI] [PubMed] [Google Scholar]
- 83.Roman D.L., Matica M.A., Ciorsac A., Boros B.V., Isvoran A. The effects of the fungicide myclobutanil on soil enzyme activity. Agriculture. 2023;13:1956. doi: 10.3390/agriculture13101956. [DOI] [Google Scholar]
- 84.Gao X., Peng Q., Yuan K., Li Y., Shi M., Miao J., Liu X. Monitoring and characterization of prochloraz resistance in Fusarium fujikuroi in China. Pestic. Biochem. Physiol. 2022;187:105189. doi: 10.1016/j.pestbp.2022.105189. [DOI] [PubMed] [Google Scholar]
- 85.Ju C., Li X., He S., Shi L., Yu S., Wang F., Xu S., Cao D., Fang H., Yu Y. Root uptake of Imidacloprid and Propiconazole is affected by root composition and soil characteristics. J. Agric. Food. Chem. 2020;68:15381–15389. doi: 10.1021/acs.jafc.0c02170. [DOI] [PubMed] [Google Scholar]
- 86.Muñoz-Leoz B., Ruiz-Romera E., Antigüedad I., Garbisu C. Tebuconazole application decreases soil microbial biomass and activity. Soil Biol. Biochem. 2011;43:2176–2183. doi: 10.1016/j.soilbio.2011.07.001. [DOI] [Google Scholar]
- 87.Dong B. A comprehensive review on toxicological mechanisms and transformation products of tebuconazole: Insights on pesticide management. Sci. Total Environ. 2023;908:168264. doi: 10.1016/j.scitotenv.2023.168264. [DOI] [PubMed] [Google Scholar]
- 88.Yu S., Li X., He S., Zhang H., Jin M., Zheng Y., Zhang L., Yu Y. Uptake and translocation of triadimefon by wheat (Triticum aestivum L.) grown in hydroponics and soil conditions. J. Hazard. Mater. 2022;423:127011. doi: 10.1016/j.jhazmat.2021.127011. [DOI] [PubMed] [Google Scholar]
- 89.Park J., Hong T., An G., Park H., Song G., Lim W. Triadimenol promotes the production of reactive oxygen species and apoptosis with cardiotoxicity and developmental abnormalities in zebrafish. Sci. Total Environ. 2023;862:160761. doi: 10.1016/j.scitotenv.2022.160761. [DOI] [PubMed] [Google Scholar]
- 90.Roman D.L., Voiculescu D.I., Matica M.A., Baerle V., Filimon M.N., Ostafe V., Isvoran A. Assessment of the effects of triticonazole on soil and human health. Molecules. 2022;27:6554. doi: 10.3390/molecules27196554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Draskau M.K., Boberg J., Taxvig C., Pedersen M., Frandsen H.L., Christiansen S., Svingen T. In vitro and in vivo endocrine disrupting effects of the azole fungicides triticonazole and flusilazole. Environ. Pollut. 2019;255:113309. doi: 10.1016/j.envpol.2019.113309. [DOI] [PubMed] [Google Scholar]
- 92.Kirman C., Li A., Sheehan P., Bus J., Lewis R., Hays S. Ethylene oxide review: Characterization of total exposure via endogenous and exogenous pathways and their implications to risk assessment and risk management. J. Toxicol. Environ. Health Sci. 2021;24:1–29. doi: 10.1080/10937404.2020.1852988. [DOI] [PubMed] [Google Scholar]
- 93.Vasamsetti B.M.K., Chon K., Yoon C.-Y., Kim J., Choi J.-Y., Hwang S., Park K.-H. Transcriptome profiling of etridiazole-exposed zebrafish (Danio rerio) embryos reveals pathways associated with cardiac and ocular toxicities. Int. J. Mol. Sci. 2023;24:15067. doi: 10.3390/ijms242015067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ullah R., Salcedo Y.E., Mukesh Mehta J., Al Balushi J., Khariwal M., Patel N. Exploring the protective role of G6PD deficiency in aluminum phosphide poisoning: A case report and review of the literature. Cureus. 2024;16:e58888. doi: 10.7759/cureus.58888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Adel B., Elgharbawy N.M., Shahin M.M., Abo-Elfadl A.A., Saad K.M. Insulin-euglycemia therapy in acute aluminum phosphide poisoning: A randomized clinical trial. Clin. Toxicol. 2023;61:1032–1039. doi: 10.1080/15563650.2023.2279495. [DOI] [PubMed] [Google Scholar]
- 96.Yang Y., Bustani G.S., Alawsi T., Altalbawy F.M.A., Kareem A.K., Gupta J., Zhu P., Hjazi A., Alawadi A.H., Mustafa Y.F. The cardioprotective effects of cerium oxide nanoparticles against the poisoning generated by aluminum phosphide pesticide: Controlling oxidative stress and mitochondrial damage. Pestic. Biochem. Phys. 2023;197:105701. doi: 10.1016/j.pestbp.2023.105701. [DOI] [PubMed] [Google Scholar]
- 97.Liman R., Ali M.M., Ciğerci İ.H., İstifli E.S., Sarıkurkcu C. Cytotoxic and genotoxic evaluation of copper oxychloride through Allium test and molecular docking studies. Environ. Sci. Pollut. Res. Int. 2021;28:44998–45008. doi: 10.1007/s11356-021-13897-4. [DOI] [PubMed] [Google Scholar]
- 98.Forouzandeh A., Blavi L., Abdelli N., Melo-Duran D., Vidal A., Rodríguez M., Monteiro A.N.T.R., Perez J.F., Darwich L., Solà-Oriol D. Effects of dicopper oxide and copper sulfate on growth performance and gut microbiota in broilers. Poult. Sci. 2021;100:101224. doi: 10.1016/j.psj.2021.101224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang T., Shi X., Wu Z., Zhang J., Hao J., Liu P., Liu X. Carboxylesterase and cytochrome P450 confer metabolic resistance simultaneously to azoxystrobin and some other fungicides in Botrytis cinerea. J. Agric. Food Chem. 2024;72:9680–9690. doi: 10.1021/acs.jafc.4c02409. [DOI] [PubMed] [Google Scholar]
- 100.Bai S., Zhang M., Tang S., Li M., Wu R., Wan S., Chen L., Wei X., Li F. Research progress on benzimidazole fungicides: A review. Molecules. 2024;29:1218. doi: 10.3390/molecules29061218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhou T., Guo T., Wang Y., Wang A., Zhang M. Carbendazim: Ecological risks, toxicities, degradation pathways and potential risks to human health. Chemosphere. 2023;314:137723. doi: 10.1016/j.chemosphere.2022.137723. [DOI] [PubMed] [Google Scholar]
- 102.Park J., An G., Park H., Hong T., Lim W., Song G. Developmental defects induced by thiabendazole are mediated via apoptosis, oxidative stress and alteration in PI3K/Akt and MAPK pathways in zebrafish. Environ. Int. 2023;176:107973. doi: 10.1016/j.envint.2023.107973. [DOI] [PubMed] [Google Scholar]
- 103.Jia K., Cheng B., Huang L., Xiao J., Bai Z., Liao X., Cao Z., Shen T., Zhang C., Hu C., et al. Thiophanate-methyl induces severe hepatotoxicity in zebrafish. Chemosphere. 2020;248:125941. doi: 10.1016/j.chemosphere.2020.125941. [DOI] [PubMed] [Google Scholar]
- 104.Ghodke P.P., Gonzalez-Vasquez G., Wang H., Johnson K.M., Sedgeman C.A., Guengerich F.P. Enzymatic bypass of an N6-deoxyadenosine DNA–ethylene dibromide–peptide cross-link by translation DNA polymerases. J. Biol. Chem. 2021;296:100444. doi: 10.1016/j.jbc.2021.100444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Park M.-G., Choi J., Hong Y.-S., Park C.G., Kim B.-G., Lee S.-Y., Lim H.J., Mo H.H., Lim E., Cha W. Negative effect of methyl bromide fumigation work on the central nervous system. PLoS ONE. 2020;15:e0236694. doi: 10.1371/journal.pone.0236694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pesonen M., Vähäkangas K. Chloropicrin-induced toxicity in the respiratory system. Toxicol. Let. 2020;323:10–18. doi: 10.1016/j.toxlet.2020.01.022. [DOI] [PubMed] [Google Scholar]
- 107.Zhu J., Cao A., Wu J., Fang W., Huang B., Yan D., Wang Q., Li Y. Effects of chloropicrin fumigation combined with biochar on soil bacterial and fungal communities and Fusarium oxysporum. Ecotoxicol. Environ. Saf. 2021;220:112414. doi: 10.1016/j.ecoenv.2021.112414. [DOI] [PubMed] [Google Scholar]
- 108.Cheng H., Zhang D., Huang B., Song Z., Ren L., Hao B., Liu J., Zhu J., Fang W., Yan D., et al. Organic fertilizer improves soil fertility and restores the bacterial community after 1,3-dichloropropene fumigation. Sci. Total Environ. 2020;738:140345. doi: 10.1016/j.scitotenv.2020.140345. [DOI] [PubMed] [Google Scholar]
- 109.Moreau M., Fisher J., Andersen M.E., Barnwell A., Corzine S., Ranade A., McMullen P.D., Slattery S.D. NAM-based prediction of point-of-contact toxicity in the lung: A case example with 1,3-dichloropropene. Toxicology. 2022;481:153340. doi: 10.1016/j.tox.2022.153340. [DOI] [PubMed] [Google Scholar]
- 110.Pandey J.K. Use of hazardous chemical pesticides in India: A review. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2023;93:523–537. doi: 10.1007/s40011-022-01425-4. [DOI] [Google Scholar]
- 111.Saquib Q., Siddiqui M.A., Ansari S.M., Alwathnani H.A., Musarrat J., Al-Khedhairy A.A. Cytotoxicity and genotoxicity of methomyl, carbaryl, metalaxyl, and pendimethalin in human umbilical vein endothelial cells. J. Appl. Toxicol. 2021;41:832–846. doi: 10.1002/jat.4139. [DOI] [PubMed] [Google Scholar]
- 112.Othman A.M., Elsayed A.M. Microbial bioremediation of fungicides. In: Abd-Elsalam K.A., editor. Nanohybrid Fungicides. Elsevier; Amsterdam, The Netherlands: 2024. pp. 441–474. [DOI] [Google Scholar]
- 113.Zia R., Taj A., Younis S., Bukhari S.Z., Latif F., Feroz Y., Midrarullah Imran A., Bajwa S.Z. Application of nanosensors for pesticide detection. In: Denizli A., Nguyen A., Nadda A.K., editors. Nanosensors for Smart Agriculture. Micro and Nano Technologies. Elsevier; Amsterdam, The Netherlands: 2024. pp. 259–302. [DOI] [Google Scholar]
- 114.Hamed S.M., Okla M.K., Al-Saadi L.S., Hozzein W.N., Mohamed H.S., Selim S., AbdElgawad H. Evaluation of the phycoremediation potential of microalgae for captan removal: Comprehensive analysis on toxicity, detoxification and antioxidants modulation. J. Hazard. Mater. 2022;427:128177. doi: 10.1016/j.jhazmat.2021.128177. [DOI] [PubMed] [Google Scholar]
- 115.Sułowicz S., Markowicz A., Dulski M., Nowak A., Środek D., Borymski S. Assessment of the ecotoxicological impact of captan@ZnO35–45nm and captan@SiO2 20–30 nm nanopesticide on non-target soil microorganisms—A 100-day case study. Appl. Soil Ecol. 2023;184:104789. doi: 10.1016/j.apsoil.2022.104789. [DOI] [Google Scholar]
- 116.Zhu Y., Ke M., Yu Z., Lei C., Liu M., Yang Y., Lu T., Zhou N.-Y., Peijnenburg W.J.G.M., Tang T., et al. Combined effects of azoxystrobin and oxytetracycline on rhizosphere microbiota of Arabidopsis thaliana. J. Environ. Int. 2024;186:108655. doi: 10.1016/j.envint.2024.108655. [DOI] [PubMed] [Google Scholar]
- 117.Chen L., Luo Y., Zhang C., Liu X., Fang N., Wang X., Zhao X., Jiang J. Trifloxystrobin induced developmental toxicity by disturbing the ABC transporters, carbohydrate and lipid metabolism in adult zebrafish. Chemosphere. 2024;349:140747. doi: 10.1016/j.chemosphere.2023.140747. [DOI] [PubMed] [Google Scholar]
- 118.Man Y., Wu C., Yu B., Mao L., Zhu L., Zhang L., Zhang Y., Jiang H., Yuan S., Zheng Y., et al. Abiotic transformation of kresoxim-methyl in aquatic environments: Structure elucidation of transformation products by LC-HRMS and toxicity assessment. Water Res. 2023;233:119723. doi: 10.1016/j.watres.2023.119723. [DOI] [PubMed] [Google Scholar]
- 119.Wu M., Bian J., Han S., Zhang C., Xu W., Tao L., Li Z., Zhang Y. Characterization of hepatotoxic effects induced by pyraclostrobin in human HepG2 cells and zebrafish larvae. Chemosphere. 2023;340:139732. doi: 10.1016/j.chemosphere.2023.139732. [DOI] [PubMed] [Google Scholar]
- 120.Xu G., Jia X., Wu C., Liu X., Dong F. Chiral fungicide famoxadone: Stereoselective bioactivity, aquatic toxicity, and environmental behavior in soils. J. Agric. Food Chem. 2021;69:8530–8535. doi: 10.1021/acs.jafc.1c00825. [DOI] [PubMed] [Google Scholar]
- 121.Li S., Geng Y., Bao C., Mei Q., Shi T., Ma X., Hua R., Fang L. Complete biodegradation of fungicide carboxin and its metabolite aniline by Delftia sp. HFL-1. Sci. Total Environ. 2024;912:168957. doi: 10.1016/j.scitotenv.2023.168957. [DOI] [PubMed] [Google Scholar]
- 122.Balasubramanya R.H., Patil R.B. Degradation of carboxin and oxycarboxin in different soils. Plant Soil. 1980;57:195–201. doi: 10.1007/BF02211679. [DOI] [Google Scholar]
- 123.Directive 2009/128/EC of the European Parliament and of the Council of 21 October 2009 Establishing a Framework for Community Action to Achieve the Sustainable Use of Pesticides. European Union; Maastricht, The Netherlands: 2009. [(accessed on 4 August 2024)]. Available online: http://data.europa.eu/eli/dir/2009/128/oj. [Google Scholar]
- 124.EU Pesticide Approvals, Renewals, and Extensions in 2023. European Union; Maastricht, The Netherlands: 2023. [(accessed on 27 May 2024)]. Regulation 2023/114. Available online: https://agrinfo.eu/book-of-reports/EU-pesticide-approvals-renewals-and-extensions-in-2023. [Google Scholar]
- 125.EU Pesticide Approvals, Renewals, and Extensions in 2023. European Union; Maastricht, The Netherlands: 2023. [(accessed on 27 May 2024)]. Regulation 2023/689. Available online: https://agrinfo.eu/book-of-reports/EU-pesticide-approvals-renewals-and-extensions-in-2023. [Google Scholar]
- 126.EU Pesticide Approvals, Renewals, and Extensions in 2023. European Union; Maastricht, The Netherlands: 2023. [(accessed on 27 May 2024)]. Regulation 2023/918. Available online: https://agrinfo.eu/book-of-reports/EU-pesticide-approvals-renewals-and-extensions-in-2023. [Google Scholar]
- 127.EU Pesticide Approvals, Renewals, and Extensions in 2023. European Union; Maastricht, The Netherlands: 2023. [(accessed on 27 May 2024)]. Regulation 2023/1446. Available online: https://agrinfo.eu/book-of-reports/EU-pesticide-approvals-renewals-and-extensions-in-2023. [Google Scholar]
- 128.EU Pesticide Approvals, Renewals, and Extensions in 2023. European Union; Maastricht, The Netherlands: 2023. [(accessed on 27 May 2024)]. Regulation 2023/2592. Available online: https://agrinfo.eu/book-of-reports/EU-pesticide-approvals-renewals-and-extensions-in-2023. [Google Scholar]
- 129.Fang W., Peng Y., Muir D., Lin J., Zhang X. A critical review of synthetic chemicals in surface waters of the US, the EU and China. Environ. Int. 2019;131:104994. doi: 10.1016/j.envint.2019.104994. [DOI] [PubMed] [Google Scholar]
- 130.Komárek M., Čadková E., Chrastný V., Bordas F., Bollinger J.C. Contamination of vineyard soils with fungicides: A review of environmental and toxicological aspects. Environ. Int. 2010;36:138–151. doi: 10.1016/j.envint.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 131.Zhang L., Zuo Q., Cai H., Li S., Shen Z., Song T. Fungicides reduce soil microbial diversity, network stability and complexity in wheat fields with different disease resistance. Appl. Soil Ecol. 2024;201:105513. doi: 10.1016/j.apsoil.2024.105513. [DOI] [Google Scholar]
- 132.Pimentão A.R., Cuco A.P., Pascoal C., Cássio F., Castro B.B. Current trends and mismatches on fungicide use and assessment of the ecological effects in freshwater ecosystems. Environ. Pollut. 2024;347:123678. doi: 10.1016/j.envpol.2024.123678. [DOI] [PubMed] [Google Scholar]
- 133.Pyatina S.A., Shishatskaya E.I., Dorokhin A.S., Menzyanova N.G. Border cell population size and oxidative stress in the root apex of Triticum aestivum seedlings exposed to fungicides. Environ. Sci. Pollut. Res. 2024;31:25600–25615. doi: 10.1007/s11356-024-32840-x. [DOI] [PubMed] [Google Scholar]
- 134.Feng Y., Huang Y., Zhan H., Bhatt P., Chen S. An overview of strobilurin fungicide degradation: Current status and future perspective. Front. Microbiol. 2020;11:389. doi: 10.3389/fmicb.2020.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Berger S., El Chazli Y., Babu A.F., Coste A.T. Azole resistance in Aspergillus fumigatus: A consequence of antifungal use in agriculture? Front. Microbiol. 2017;8:1024. doi: 10.3389/fmicb.2017.01024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rani A., Singh R., Kumar P., Shukla G. Pros and cons of fungicides: An overwiev. Int. J. Eng. Sci. Res. Technol. 2018;6:112–117. doi: 10.5281/zenodo.233295. [DOI] [Google Scholar]
- 137.Wang M., Duan H., Zhou C., Yu L., Meng X., Lu W., Yu H. Synergistic effects of chemical fungicides with crude extracts from Bacillus amyloliquefaciens to control northern corn leaf blight. Agriculture. 2024;14:606. doi: 10.3390/agriculture14040606. [DOI] [Google Scholar]
- 138.Stenberg J.A., Sundh I., Becher P.G., Björkman C., Dubey M., Egan P.A., Friberg H., Gil J.F., Jensen D.F., Jonsson M., et al. When is it biological control? A framework of definitions, mechanisms, and classifications. J. Pest Sci. 2021;94:665–676. doi: 10.1007/s10340-021-01354-7. [DOI] [Google Scholar]
- 139.Robin D.C., Marchand P.A. Evolution of the biocontrol active substances in the framework of the European Pesticide Regulation (EC) No. 1107/2009. Pest Manag. Sci. 2019;75:950–958. doi: 10.1002/ps.5199. [DOI] [PubMed] [Google Scholar]
- 140.Marchand P.A. BioControl Agents in Europe: Substitution plant protection active substances or a new paradigm? Agrochemicals. 2023;2:538–550. doi: 10.3390/agrochemicals2040030. [DOI] [Google Scholar]
- 141.Eilenberg J., Hajek A., Lomer C. Suggestions for unifying the terminology in biological control. Biocontrol. 2001;46:387–400. doi: 10.1023/A:1014193329979. [DOI] [Google Scholar]
- 142.Heimpel G.E., Mills N.J. Biological Control—Ecology and Applications. Cambridge University Press; Cambridge, UK: 2017. [DOI] [Google Scholar]
- 143.Galli M., Feldmann F., Vogler U.K., Kogel K.H. Can biocontrol be the game-changer in integrated pest management? A review of definitions, methods and strategies. J. Plant Dis. Prot. 2024;131:265–291. doi: 10.1007/s41348-024-00878-1. [DOI] [Google Scholar]
- 144.Fuerst E.P., James M.S., Pollard A.T., Okubara P.A. Defense enzyme responses in dormant wild oat and wheat caryopses challenged with a seed decay pathogen. Front. Plant Sci. 2018;8:2259. doi: 10.3389/fpls.2017.02259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bailly C., Bousquet A., Braun V., Buitink J., Desbois-Vimont C., Durand Tardif M., Fougereux J.A., Gaillard A., Gouleau A., Grappin P., et al. Towards Seed Protection Using Biocontrol Strategies. HAL Open Science; Paris, France: 2019. [(accessed on 24 May 2024)]. hal-02931599. Available online: https://hal.science/hal-02931599. [Google Scholar]
- 146.O’Callaghan M. Microbial inoculation of seed for improved crop performance: Issues and opportunities. Appl. Microbiol. Biotechnol. 2016;100:5729–5746. doi: 10.1007/s00253-016-7590-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rocha I., Ma Y., Souza-Alonso P., Vosátka M., Freitas H., Oliveira R.S. Seed coating: A tool for delivering beneficial microbes to agricultural crops. Front. Plant Sci. 2019;10:1357. doi: 10.3389/fpls.2019.01357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kaufman G. Seed coating: A tool for stand establishment; a stimulus to seed quality. HortTechnology. 1991;1:98–102. doi: 10.21273/HORTTECH.1.1.98. [DOI] [Google Scholar]
- 149.Bakker P.A., Pieterse C.M., Van Loon L.C. Induced systemic resistance by fluorescent Pseudomonas spp. Phytopathology. 2007;97:239–243. doi: 10.1094/PHYTO-97-2-0239. [DOI] [PubMed] [Google Scholar]
- 150.Newitt J.T., Prudence S.M.M., Hutchings M.I., Worsley S.F. Biocontrol of cereal crop diseases using Streptomycetes. Pathogens. 2019;8:78. doi: 10.3390/pathogens8020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chan Y.K., Savard M.E., Reid L.M., Cyr T., McCormick W.A., Seguin C. Identification of lipopeptide antibiotics of a Bacillus subtilis isolate and their control of Fusarium graminearum diseases in maize and wheat. BioControl. 2009;54:567–574. doi: 10.1007/s10526-008-9201-x. [DOI] [Google Scholar]
- 152.Dunlap C.A., Schisler D.A., Price N.P., Vaughn S.F. Cyclic lipopeptide profile of three Bacillus subtilis strains, antagonists of Fusarium head blight. J. Microbiol. 2011;49:603–609. doi: 10.1007/s12275-011-1044-y. [DOI] [PubMed] [Google Scholar]
- 153.Zanon M.S.A., Cavaglieri L.R., Palazzini J.M., Chulze S.N., Chiotta M.L. Bacillus velezensis RC218 and emerging biocontrol agents against Fusarium graminearum and Fusarium poae in barley: In vitro, greenhouse and field conditions. Int. J. Food Microbiol. 2024;413:110580. doi: 10.1016/j.ijfoodmicro.2024.110580. [DOI] [PubMed] [Google Scholar]
- 154.Pan D., Mionetto A., Tiscornia S., Bettucci L. Endophytic bacteria from wheat grain as biocontrol agents of Fusarium graminearum and deoxynivalenol production in wheat. Mycotoxin Res. 2015;31:137–143. doi: 10.1007/s12550-015-0224-8. [DOI] [PubMed] [Google Scholar]
- 155.Zhao Z., Wang Q., Wang K., Brian K., Liu C., Gu Y. Study of the antifungal activity of Bacillus vallismortis ZZ185 in vitro and identification of its antifungal components. Bioresour. Technol. 2010;101:292–297. doi: 10.1016/j.biortech.2009.07.071. [DOI] [PubMed] [Google Scholar]
- 156.Karačić V., Miljaković D., Marinković J., Ignjatov M., Milošević D., Tamindžić G., Ivanović M. Bacillus species: Excellent biocontrol agents against tomato fiseases. Microorganisms. 2024;12:457. doi: 10.3390/microorganisms12030457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Baffoni L., Gaggia F., Dalanaj N., Prodi A., Nipoti P., Pisi A., Biavati B., Di Gioia D. Microbial inoculants for the biocontrol of Fusarium spp. in durum wheat. BMC Microbiol. 2015;15:242. doi: 10.1186/s12866-015-0573-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhao H., Vegi A., Wolf-Hall C. Screening of lactic acid bacteria for anti-Fusarium activity and optimization of incubation conditions. J. Food Prot. 2017;80:1648–1656. doi: 10.4315/0362-028X.JFP-17-100. [DOI] [PubMed] [Google Scholar]
- 159.Rajendran K., Krishnamoorthy M., Karuppiah K., Ethiraj K., Sekar S. Chitinase from Streptomyces mutabilis as an effective eco-friendly biocontrol agent. Appl. Biochem. Biotechnol. 2024;196:18–31. doi: 10.1007/s12010-023-04489-8. [DOI] [PubMed] [Google Scholar]
- 160.Lu Y., Song W., Wang J., Cao Y., Han X., Xu C., Wang F., Ge B. Biocontrol of Botrytis cinerea by Streptomyces noursei C27 and preliminary identification of antimicrobial metabolites. Biol. Contr. 2024;196:105561. doi: 10.1016/j.biocontrol.2024.105561. [DOI] [Google Scholar]
- 161.Matić S., Spadaro D., Garibaldi A., Gullino M.L. Antagonistic yeasts and thermotherapy as seed treatments to control Fusarium fujikuroi on rice. Biol. Control. 2014;73:59–67. doi: 10.1016/j.biocontrol.2014.03.008. [DOI] [Google Scholar]
- 162.Druvefors A.U., Schnürer J. Mold-inhibitory activity of different yeast species during airtight storage of wheat grain. FEMS Yeast Res. 2005;5:373–378. doi: 10.1016/j.femsyr.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 163.Sun Z., Li S., Ren Q., Xu J., Lu X., Sun M. Biology and applications of Clonostachys rosea. J. Appl. Microbiol. 2020;129:486–495. doi: 10.1111/jam.14625. [DOI] [PubMed] [Google Scholar]
- 164.Jensen B., Knudsen I.M.B., Jensen D.F. Biological seed treatment of cereals with fresh and long-term stored formulations of Clonostachys rosea: Biocontrol efficacy against Fusarium culmorum. Eur. J. Plant Pathol. 2000;106:233–242. doi: 10.1023/A:1008794626600. [DOI] [Google Scholar]
- 165.Shi C., Yan P., Li J., Wu H., Li Q., Guan S. Biocontrol of Fusarium graminearum growth and deoxynivalenol production in wheat kernels with bacterial antagonists. Int. J. Environ. Res. Public Health. 2014;11:1094–1105. doi: 10.3390/ijerph110101094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.He J., Boland G.J., Zhou T. Concurrent selection for microbial suppression of Fusarium graminearum, Fusarium head blight and deoxynivalenol in wheat. J. Appl. Microbiol. 2009;106:1805–1817. doi: 10.1111/j.1365-2672.2009.04147.x. [DOI] [PubMed] [Google Scholar]
- 167.Nourozian J., Etebarian H.R., Khodakaramian G. Biological control of Fusarium graminearum on wheat by antagonistic bacteria. Songklanakarin J. Sci. Technol. 2006;28((Suppl. S1)):29–38. [Google Scholar]
- 168.Johnsson L., Hökeberg M., Gerhardson B. Performance of the Pseudomonas chlororaphis biocontrol agent MA 342 against cereal seed-borne diseases in field experiments. Eur. J. Plant Pathol. 1998;104:701–711. doi: 10.1023/A:1008632102747. [DOI] [Google Scholar]
- 169.Mattei V., Motta A., Saracchi M., Kunova A., Cortesi P., Pizzatti C., Pasquali M. Wheat seed coating with Streptomyces sp. strain DEF39 spores protects against Fusarium head blight. Microorganisms. 2022;10:1536. doi: 10.3390/microorganisms10081536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Nagaraja H., Chennappa G., Rakesh S., Naik M.K., Amaresh Y.S., Sreenivasa M.Y. Antifungal activity of Azotobacter nigricans against trichothecene-producing Fusarium species associated with cereals. Food Sci. Biotechnol. 2016;25:1197–1204. doi: 10.1007/s10068-016-0190-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Schisler D.A., Core A.B., Boehm M.J., Horst L., Krause C., Dunlap C.A., Rooney A.P. Population dynamics of the Fusarium head blight biocontrol agent Cryptococcus flavescens OH 182.9 on wheat anthers and heads. Biol. Contr. 2014;70:17–27. doi: 10.1016/j.biocontrol.2013.11.011. [DOI] [Google Scholar]
- 172.Xue A.G., Guo W., Chen Y., Siddiqui I., Marchand G., Liu J., Changzhong R. Effect of seed treatment with novel strains of Trichoderma spp. on establishment and yield of spring wheat. Crop Prot. 2017;96:97–102. doi: 10.1016/j.cropro.2017.02.003. [DOI] [Google Scholar]
- 173.Petros Kubheka B., Weldegabir Ziena L. production. In: Juliatti F.C., editor. Trichoderma—Technology and Uses. IntechOpen; London, UK: 2024. [DOI] [Google Scholar]
- 174.Ferrigo D., Mondin M., Edith L., Fabio F., Causin R., Raiola A. Effect of seed biopriming with Trichoderma harzianum strain INAT11 on Fusarium ear rot and Gibberella ear rot diseases. Biol. Contr. 2020;147:104286. doi: 10.1016/j.biocontrol.2020.104286. [DOI] [Google Scholar]
- 175.Modrzewska M., Bryła M., Kanabus J., Pierzgalski A. Trichoderma as a biostimulator and biocontrol agent against Fusarium in the production of cereal crops: Opportunities and Possibilities. Plant Pathol. 2022;71:1471–1485. doi: 10.1111/ppa.13578. [DOI] [Google Scholar]
- 176.Benítez T., Rincón A.M., Limón M.C., Codón A.C. Biocontrol mechanisms of Trichoderma strains. Int. Microbiol. 2004;7:249–260. [PubMed] [Google Scholar]
- 177.Kumari R., Kumar V., Arukha A.P., Rabbee M.F., Ameen F., Koul B. Screening of the biocontrol efficacy of potent Trichoderma strains against Fusarium oxysporum f. sp. ciceri and Scelrotium rolfsii causing wilt and collar rot in chickpea. Microorganisms. 2024;12:1280. doi: 10.3390/microorganisms12071280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bharti L., Yadav K., Kumar Chaubey A. Trichoderma spp.: Approach for bio-control agent. In: Bahadur A., editor. Challenges in Plant Disease Detection and Recent Advancements. IntechOpen; London, UK: 2024. [DOI] [Google Scholar]
- 179.Natsiopoulos D., Topalidou E., Mantzoukas S., Eliopoulos P.A. Endophytic Trichoderma: Potential and prospects for plant health management. Pathogens. 2024;13:548. doi: 10.3390/pathogens13070548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wachowska U., Packa D., Wiwart M. Microbial inhibition of Fusarium pathogens and biological modification of trichothecenes in cereal grains. Toxins. 2017;9:408. doi: 10.3390/toxins9120408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Larrea M., Nicole C., Benítez Rodas G.A., Sandoval-Espínola W.J., Arrúa P.D., Lopez-Nicora H.Q., Arrúa S.A., Fernández Rios D., Arrúa A.A. Trichoderma as biocontrol agent—In focus. Rev. Soc. Cient. Parag. 2024;29:137–171. doi: 10.32480/rscp.2024.29.1.137. [DOI] [Google Scholar]
- 182.Perrone G., Ferrara M., Medina A., Pascale M., Magan N. Toxigenic fungi and mycotoxins in a climate change scenario: Ecology, genomics, distribution, prediction and prevention of the risk. Microorganisms. 2020;8:1496. doi: 10.3390/microorganisms8101496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.BIOMIN Science & Solutions. BIOMIN Holding GmbH; Herzogenburg, Austria: 2015. [Google Scholar]
- 184.Ndiaye S., Zhang M., Fall M., Ayessou N.M., Zhang Q., Li P. Current review of mycotoxin biodegradation and bioadsorption: Microorganisms, mechanisms, and main important applications. Toxins. 2022;14:729. doi: 10.3390/toxins14110729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Harkai P., Szabó I., Cserháti M., Krifaton C., Risa A., Radó J., Balázs A., Berta K., Kriszt B. Biodegradation of aflatoxin-B1 and zearalenone by Streptomyces sp. collection. Int. Biodeterior. Biodegrad. 2016;108:48–56. doi: 10.1016/j.ibiod.2015.12.007. [DOI] [Google Scholar]
- 186.Błaszczyk L., Basińska-Barczak A., Ćwiek-Kupczyńska H., Gromadzka K., Popiel D., Stępień L. Suppressive effect of Trichoderma spp. on toxigenic Fusarium species. Pol. J. Microbiol. 2017;66:85–100. doi: 10.5604/17331331.1234997. [DOI] [PubMed] [Google Scholar]
- 187.Tian Y., Tan Y., Yan Z., Liao Y., Chen J., De Boevre M., De Saeger S., Wu A. Antagonistic and detoxification potentials of Trichoderma isolates for control of zearalenone (ZEN) producing Fusarium graminearum. Front. Microbiol. 2018;8:2710. doi: 10.3389/fmicb.2017.02710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Galletti S., Paris R., Cianchetta S. Selected isolates of Trichoderma gamsii induce different pathways of systemic resistance in maize upon Fusarium verticillioides challenge. Microbiol. Res. 2020;233:126406. doi: 10.1016/j.micres.2019.126406. [DOI] [PubMed] [Google Scholar]
- 189.Dini I., Alborino V., Lanzuise S., Lombardi N., Marra R., Balestrieri A., Ritieni A., Woo S.L., Vinale F. Trichoderma enzymes for degradation of aflatoxin B1 and ochratoxin A. Molecules. 2022;7:3959. doi: 10.3390/molecules27123959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Liu L., Xie M., Wei D. Biological detoxification of mycotoxins: Current status and future advances. Int. J. Mol. Sci. 2022;23:1064. doi: 10.3390/ijms23031064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Vinderola G., Ritieni A. Role of probiotics against mycotoxins and their deleterious effects. J. Food Res. 2014;4:10–21. doi: 10.5539/jfr.v4n1p10. [DOI] [Google Scholar]
- 192.Sangsila A., Faucet-Marquis V., Pfohl-Leszkowicz A., Itsaranuwat P. Detoxification of zearalenone by Lactobacillus pentosus strains. Food Contr. 2016;62:187–192. doi: 10.1016/j.foodcont.2015.10.031. [DOI] [Google Scholar]
- 193.Tinyiro S.E., Wokadala C., Xu D., Yao W. Adsorption and degradation of zearalenone by Bacillus strains. Folia Microbiol. 2011;56:321–327. doi: 10.1007/s12223-011-0047-8. [DOI] [PubMed] [Google Scholar]
- 194.Farzaneh M., Shi Z.-Q., Ghassempour A., Sedaghat N., Ahmadzadeh M., Mirabolfathy M., Javan-Nikkhah M. Aflatoxin B1 degradation by Bacillus subtilis UTBSP1 isolated from pistachio nuts of Iran. Food Contr. 2021;23:100–106. doi: 10.1016/j.foodcont.2011.06.018. [DOI] [Google Scholar]
- 195.Zeidan R., Ul Hassan Z., Ashfaq M.Y., Al-Thani R., Jaoua S. Investigation of heat-resistant antifungal agents from Bacillus amyloliquefaciens and Bacillus subtilis for biocontrol of mycotoxigenic fungi. Environ. Technol. Innov. 2024;36:103748. doi: 10.1016/j.eti.2024.103748. [DOI] [Google Scholar]
- 196.Yang X., Zhang Q., Chen Z.Y., Liu H., Li P. Investigation of Pseudomonas fluorescens strain 3JW1 on preventing and reducing aflatoxin contaminations in peanuts. PLoS ONE. 2017;12:e0178810. doi: 10.1371/journal.pone.0178810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Jakopović Ž., Čiča K.H., Mrvčić J., Pucić I., Čanak I., Frece J., Pleadin J., Stanzer D., Zjalić S., Markov K. Properties and fermentation activity of industrial yeasts Saccharomyces cerevisiae, S. uvarum, Candida utilis and Kluyveromyces marxianus exposed to AFB1, OTA and ZEA. Food Technol. Biotechnol. 2018;56:208–217. doi: 10.17113/ftb.56.02.18.5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Adeleke B.S., Ayilara M.S., Akinola S.A., Babalola O.O. Biocontrol mechanisms of endophytic fungi. Egypt. J. Biol. Pest Control. 2022;32:46. doi: 10.1186/s41938-022-00547-1. [DOI] [Google Scholar]
- 199.Fenta L., Mekonnen H. Microbial biofungicides as a substitute for chemical fungicides in the control of phytopathogens: Current perspectives and research directions. Scientifica. 2024;2024:5322696. doi: 10.1155/2024/5322696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Goettel M.S., Hajek A.E., Siegel J.P., Evans H.C. Safety of fungal biocontrol agents. In: Butt T.M., Jackson C., Magan N., editors. Fungi as Biocontrol Agents: Progress, Problems and Potential. CABI International; Wallingford, UK: 2001. pp. 347–375. [DOI] [Google Scholar]
- 201.Bonaterra A., Badosa E., Cabrefiga J., Frances J., Montesinos E. Prospects and limitations of microbial pesticides for control of bacterial and fungal pomefruit tree diseases. Trees. 2012;26:215–226. doi: 10.1007/s00468-011-0626-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Sal E., Stemler J., Salmanton-García J., Falces-Romero I., Kredics L., Meyer E., Würstl B., Lass-Flörl C., Racil Z., Klimko N., et al. Invasive Trichoderma spp. infections: Clinical presentation and outcome of cases from the literature and the FungiScope® registry. J. Antimicrob. Chemother. 2022;77:2850–2858. doi: 10.1093/jac/dkac235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Prakash B., Singh P., Kedia A., Dubey N.K. Assessment of some essential oils as food preservatives based on antifungal, antiaflatoxin, antioxidant activities and in vivo efficacy in food system. Int. Food Res. 2012;49:201–208. doi: 10.1016/j.foodres.2012.08.020. [DOI] [Google Scholar]
- 204.Taheri P., Soweizy M., Tarighi S. Application of essential oils to control some important fungi and bacteria pathogenic on cereals. J. Nat. Pest. Res. 2023;6:100052. doi: 10.1016/j.napere.2023.100052. [DOI] [Google Scholar]
- 205.Uwineza P.A., Urbaniak M., Stępień Ł., Gramza-Michałowska A., Waśkiewicz A. Efficacy of Lamium album as a natural fungicide: Impact on seed germination, ergosterol, and mycotoxins in Fusarium culmorum-infected wheat seedlings. Front. Microbiol. 2024;15:1363204. doi: 10.3389/fmicb.2024.1363204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ben Miri Y., Nouasri A., Herrera M., Djenane D., Ariño A. Antifungal activity of menthol, eugenol and their combination against Aspergillus ochraceus and Aspergillus niger in vitro and in stored cereals. Foods. 2023;12:2108. doi: 10.3390/foods12112108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Almeida N.A., Freire L., Carnielli-Queiroz L., Bragotto A.P.A., Silva N.C.C., Rocha L.O. Essential oils: An eco-friendly alternative for controlling toxigenic fungi in cereal grains. Compr. Rev. Food Sci. Food Saf. 2024;23:e13251. doi: 10.1111/1541-4337.13251. [DOI] [PubMed] [Google Scholar]
- 208.Kursa W., Jamiołkowska A., Wyrostek J., Kowalski R. Antifungal effect of plant extracts on the growth of the cereal pathogen Fusarium spp.—An in vitro study. Agronomy. 2022;12:3204. doi: 10.3390/agronomy12123204. [DOI] [Google Scholar]
- 209.Gwad M.M.A., El-Sayed A.S.A., Abdel-Fattah G.M., Abdelmoteleb M., Abdel-Fattah G.G. Potential fungicidal and antiaflatoxigenic effects of cinnamon essential oils on Aspergillus flavus inhabiting the stored wheat grains. BMC Plant Biol. 2024;24:394. doi: 10.1186/s12870-024-05065-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Anžlovar S., Likar M., Koce J.D. Antifungal potential of thyme essential oil as a preservative for storage of wheat seeds. Acta Bot. Croat. 2017;761:64–71. doi: 10.1515/botcro-2016-0044. [DOI] [Google Scholar]
- 211.Belasli A., Miri Y.B., Aboudaou M., Ouahioune L.A., Montañes L., Ariño A., Djenane D. Antifungal, antitoxigenic, and antioxidant activities of the essential oil from laurel (Laurus nobilis L.): Potential use as wheat preservative. Food Sci. Nutr. 2020;8:4717–4729. doi: 10.1002/fsn3.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lee L.T., Martinazzo A.P., Garcia S.A., Berbet P.A., Teodoro C.E.S. Control of Aspergillus flavus in wheat grains using Cymbopogon flexuosus essential oil. Acta Bras. 2021;5:92–96. doi: 10.22571/2526-4338528. [DOI] [Google Scholar]
- 213.Roselló J., Sempere F., Berzola-Sanz I., Chiralt A., Santamarina M.P. Antifungal activity and potential use of essential oils against Fusarium culmorum and Fusarium verticillioides. J. Essent. Oil-Bear. Plants. 2015;18:359–367. doi: 10.1080/0972060X.2015.1010601. [DOI] [Google Scholar]
- 214.Bocate K.P., Evangelista A.G., Luciano F.B. Garlic essential oil as an antifungal and anti-mycotoxin agent in stored corn. LWT—Food Sci. Technol. 2021;147:111600. doi: 10.1016/j.lwt.2021.111600. [DOI] [Google Scholar]
- 215.Perczak A., Gwiazdowska D., Gwiazdowski R., Juś K., Marchwińska K., Waśkiewicz A. The inhibitory potential of selected essential oils on Fusarium spp. growth and mycotoxins biosynthesis in maize seeds. Pathogens. 2019;9:23. doi: 10.3390/pathogens9010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Tucker M.A., Lopez-Ruiz F., Jayasena K., Oliver R.P. Origin of Fungicide-Resistant Barley Powdery Mildew in Western Australia: Lessons to Be Learned. In: Ishii H., Hollomon D., editors. Fungicide Resistance in Plant Pathogens. Springer; Tokyo, Japan: 2015. pp. 329–340. [DOI] [Google Scholar]
- 217.Kang Z., Li X., Wan A., Wang M., Chen X. Differential sensitivity among Puccinia striiformis f. sp. tritici isolates to propiconazole and pyraclostrobin fungicides . Can. J. Plant Pathol. 2019;41:415–434. doi: 10.1080/07060661.2019.1577301. [DOI] [Google Scholar]
- 218.Vielba-Fernández A., Polonio Á., Ruiz-Jiménez L., de Vicente A., Pérez-García A., Fernández-Ortuño D. Fungicide resistance in powdery mildew fungi. Microorganisms. 2020;8:1431. doi: 10.3390/microorganisms8091431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Richard B., Qi A., Fitt B.D.L. Control of crop diseases through integrated crop management to deliver climate-smart farming systems for low- and high-input crop production. Plant Pathol. 2022;71:187–206. doi: 10.1111/ppa.13493. [DOI] [Google Scholar]
- 220.Jørgensen L.N., Matzen N., Leitzke R., Thomas J.E., O’Driscoll A., Klocke B., Maumene C., Lindell I., Wahlquist K., Zemeca L., et al. Management of rust in wheat using IPM principles and alternative products. Agriculture. 2024;14:821. doi: 10.3390/agriculture14060821. [DOI] [Google Scholar]
- 221.Ghorbani R., Wilcockson S.J., Giotis C., Leifert C. Potato late blight management in organic agriculture. Pest Manag. 2004;15:176–180. doi: 10.1564/15aug12. [DOI] [Google Scholar]
- 222.Zhang C., Wang W., Xue M., Liu Z., Zhang Q., Hou J., Xing M., Wang R., Liu T. The combination of a biocontrol agent Trichoderma asperellum SC012 and hymexazol reduces the effective fungicide dose to control Fusarium wilt in cowpea. J. Fungi. 2021;7:685. doi: 10.3390/jof7090685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Shashikumar H.M., Koulagi S., Navyashree S.E. Compatibility of Trichoderma viride and Trichoderma harzianum with Fungicides against Soil Borne Diseases of Tomato and Cabbage. Int. J. Curr. Microbiol. App. Sci. 2019;8:1920–1928. doi: 10.20546/ijcmas.2019.804.225. [DOI] [Google Scholar]
- 224.Saha S., Bhosale S., Chavan V., Thosar R., Ranade Y., Holkar S., Rai R., Kulkarni M., Kulkarni Y., Tiwari A. Study of endophytes as biocontrol agents vis-a-vis their compatibility to fungicides in grapes. Int. J. Bio-Resour. Stress Manag. 2023;14:1539–1549. doi: 10.23910/1.2023.4886a. [DOI] [Google Scholar]
- 225.Tomer A., Sigh R., Prasad D. Compatibility of Trichoderma harzianum with systemic and two non-systemic fungicides in vitro. Asian J. Crop Sci. 2018;10:174–179. doi: 10.3923/ajcs.2018.174.179. [DOI] [Google Scholar]
- 226.Madhavi G.B., Bhattiprolu S.L., Reddy V.B. Compatibility of biocontrol agent Trichoderma viride with various pesticides. J. Hortic. Sci. 2011;6:71–73. doi: 10.24154/jhs.v6i1.448. [DOI] [Google Scholar]
- 227.Escudero-Leyva E., Alfaro-Vargas P., Muñoz-Arrieta R., Charpentier-Alfaro C., Granados-Montero M.M., Valverde-Madrigal K.S., Pérez-Villanueva M., Méndez-Rivera M., Rodríguez-Rodríguez C.E., Chaverri P., et al. Tolerance and biological removal of fungicides by Trichoderma species isolated from the endosphere of wild Rubiaceae plants. Front. Agron. 2022;3:772170. doi: 10.3389/fagro.2021.772170. [DOI] [Google Scholar]
- 228.Sunkad G., Meghana P. Compatibility of indigenous Trichoderma asperellum with chemical fungicides for the management of chickpea wilt. J. Biol. Control. 2023;37:6. doi: 10.18311/jbc/2023/32435. [DOI] [Google Scholar]
- 229.Lima G., Castoria R., De Curtis F., Raiola A., Ritieni A., De Cicco V. Integrated control of blue mould using new fungicides and biocontrol yeasts lowers levels of fungicide residues and patulin contamination in apples. Postharvest Biol. Technol. 2011;60:164–172. doi: 10.1016/j.postharvbio.2010.12.010. [DOI] [Google Scholar]
- 230.Errampalli D., Brubacher N.R. Biological and integrated control of postharvest blue mold (Penicillium expansum) of apples by Pseudomonas syringae and cyprodinil. Biol. Control. 2006;36:49–56. doi: 10.1016/j.biocontrol.2005.07.011. [DOI] [Google Scholar]
- 231.Musheer N., Jamil A., Choudhary A. Solo and combined applications of fungicides and bio-agents to reduce severity of Fusarium oxysporum and induce antioxidant metabolites in Ocimum tenuiflorum L. J. Plant Pathol. 2023;105:237–251. doi: 10.1007/s42161-022-01271-3. [DOI] [Google Scholar]
- 232.Palazzini J.M., Torres A.M., Chulze S.N. Tolerance of triazole-based fungicides by biocontrol agents used to control Fusarium head blight in wheat in Argentina. Lett. Appl. Microbiol. 2018;66:434–438. doi: 10.1111/lam.12869. [DOI] [PubMed] [Google Scholar]
- 233.Curtis F.D., De Cicco V., Lima G. Efficacy of biocontrol yeasts combined with calcium silicate or sulphur for controlling durum wheat powdery mildew and increasing grain yield components. Field Crops Res. 2012;134:36–46. doi: 10.1016/j.fcr.2012.04.014. [DOI] [Google Scholar]
- 234.Fielding B.C., Knowles C., Vries F.A., Klaasen J.A. Testing of eight medicinal plant extracts in combination with kresoxim-methyl for integrated control of Botrytis cinerea in apples. Agriculture. 2015;5:400–411. doi: 10.3390/agriculture5030400. [DOI] [Google Scholar]
- 235.Adandonon A., Aveling T.A.S., Labuschagne N., Tamo M. Biocontrol agents in combination with Moringa oleifera extract for integrated control of Sclerotium-caused cowpea damping-off and stem rot. Eur. J. Plant Pathol. 2006;115:409–418. doi: 10.1007/s10658-006-9031-6. [DOI] [Google Scholar]
- 236.El-kazzaz M.K., Salem E.A., Ghoneim K.E., Elsharkawy M.M., El-kot G.A.E.N. Integrated control of rice kernel smut disease using plant extracts and salicylic acid. Arch. Phytopathol. Plant Prot. 2015;48:664–675. doi: 10.1080/03235408.2015.1092202. [DOI] [Google Scholar]
- 237.Panday D., Bhusal N., Das S., Ghalehgolabbehbahani A. Rooted in nature: The rise, challenges, and potential of organic farming and fertilizers in agroecosystems. Sustainability. 2024;16:1530. doi: 10.3390/su16041530. [DOI] [Google Scholar]
- 238.Mihelič R., Pintarič S., Eler K., Suhadolc M. Effects of transitioning from conventional to organic farming on soil organic carbon and microbial community: A comparison of long-term non-inversion minimum tillage and conventional tillage. Biol. Fertil. Soils. 2024;60:341–355. doi: 10.1007/s00374-024-01796-y. [DOI] [Google Scholar]
- 239.Burandt Q.C., Deising H.B., von Tiedemann A. Further limitations of synthetic fungicide use and expansion of Organic Agriculture in Europe Will Increase the Environmental and Health Risks of Chemical Crop Protection Caused by copper-containing fungicides. Environ. Toxicol Chem. 2024;43:19–30. doi: 10.1002/etc.5766. [DOI] [PubMed] [Google Scholar]
- 240.Soussi A., Zero E., Sacile R., Trinchero D., Fossa M. Smart sensors and smart data for precision agriculture: A Review. Sensors. 2024;24:2647. doi: 10.3390/s24082647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Salas B., Salcedo R., Garcia-Ruiz F., Gil E. Design, implementation and validation of a sensor-based precise airblast sprayer to improve pesticide applications in orchards. Precis. Agric. 2024;25:865–888. doi: 10.1007/s11119-023-10097-7. [DOI] [Google Scholar]
- 242.Santos J.L., Pereira P.S., Reis K.H.B., Freitas D.R., Picanço Filho M.C., Peluzio J.M., Sarmento R.A., Guedes R.N.C., Picanço M.C. Decision-making for thrips control in soybean fields using precision agriculture principles. J. Appl. Entomol. 2024;148:140–149. doi: 10.1111/jen.13215. [DOI] [Google Scholar]
- 243.Mehedi I.M., Hanif M.S., Bilal M., Vellingiri M.T., Palaniswamy T. Remote sensing and decision support system applications in precision agriculture: Challenges and possibilities. IEEE Access. 2024;12:44786–44798. doi: 10.1109/ACCESS.2024.3380830. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.