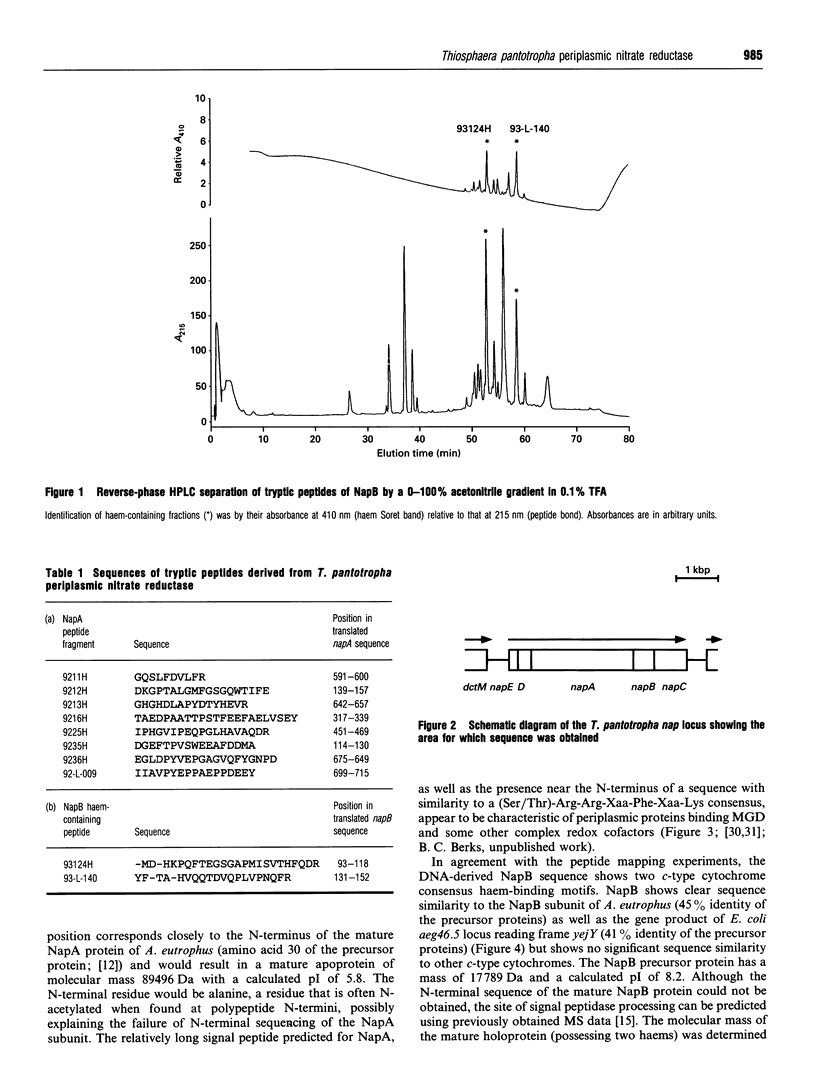
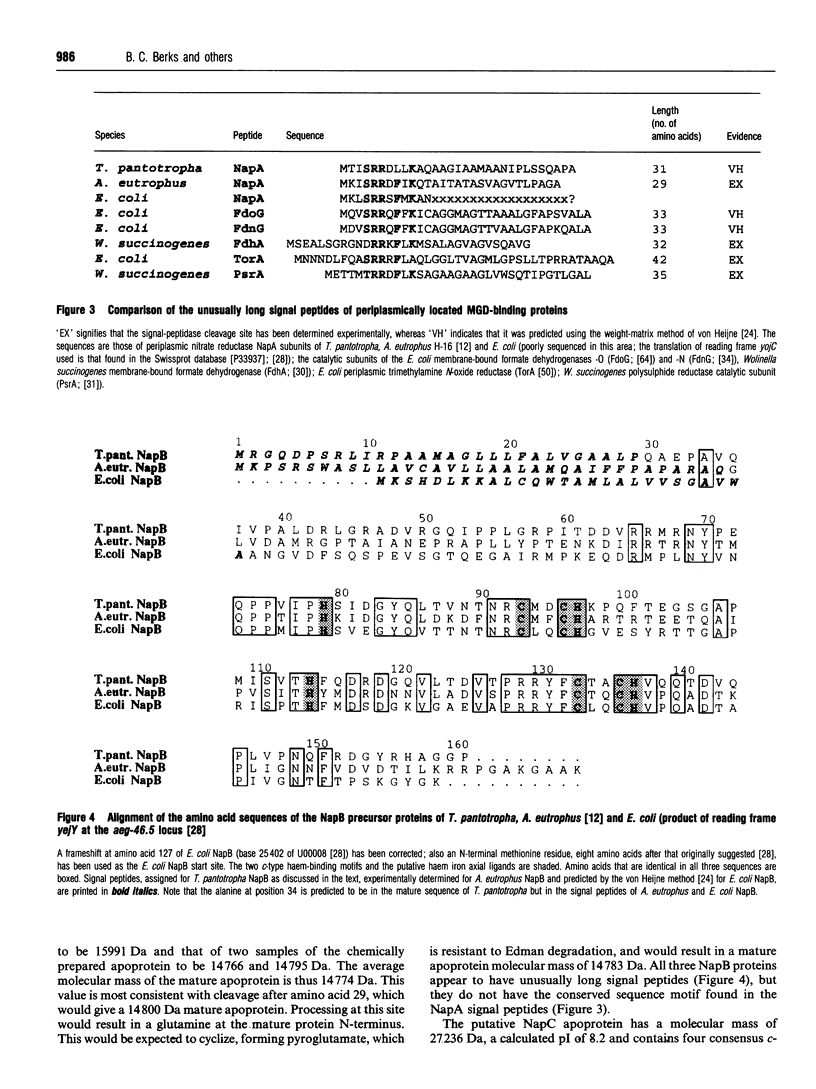
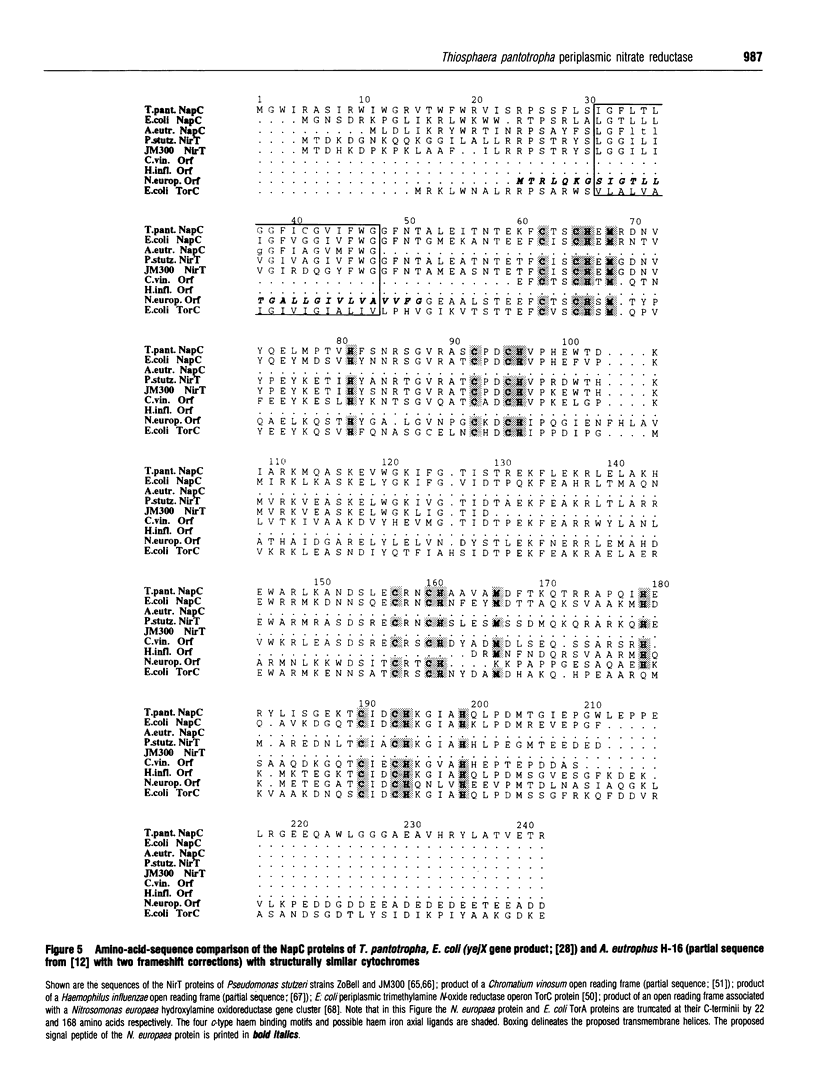
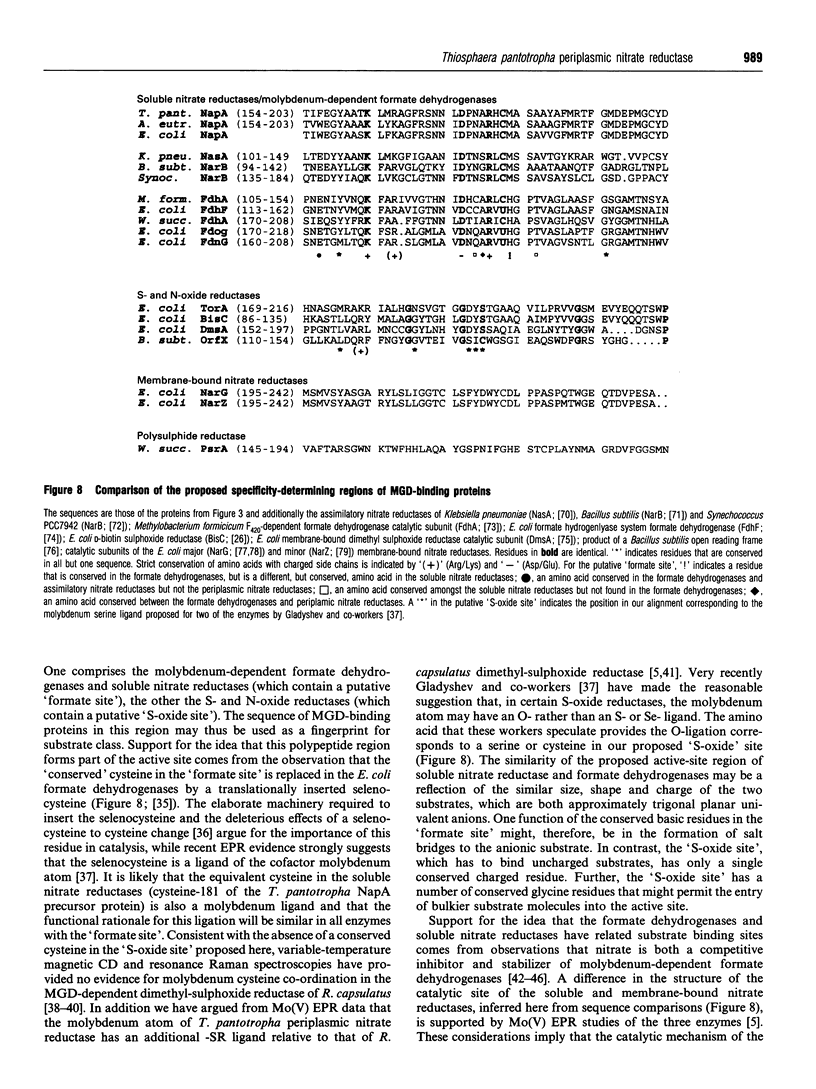
Abstract
The napEDABC locus coding for the periplasmic nitrate reductase of Thiosphaera pantotropha has been cloned and sequenced. The large and small subunits of the enzyme are coded by napA and napB. The sequence of NapA indicates that this protein binds the GMP-conjugated form of the molybdopterin cofactor. Cysteine-181 is proposed to ligate the molybdenum atom. It is inferred that the active site of the periplasmic nitrate reductase is structurally related to those of the molybdenum-dependent formate dehydrogenases and bacterial assimilatory nitrate reductases, but is distinct from that of the membrane-bound respiratory nitrate reductases. A four-cysteine motif at the N-terminus of NapA binds a [4Fe-4S] cluster. The DNA- and protein-derived primary sequence of NapB confirm that this protein is a dihaem c-type cytochrome and, together with spectroscopic data, indicate that both NapB haems have bis-histidine ligation. napC is predicted to code for a membrane-anchored tetrahaem c-type cytochrome that shows sequence similarity to the NirT cytochrome c family. NapC may be the direct electron donor to the NapAB complex. napD is predicted to encode a soluble cytoplasmic protein and napE a monotopic integral membrane protein, napDABC genes can be discerned at the aeg-46.5 locus of Escherichia coli K-12, suggesting that this operon encodes a periplasmic nitrate reductase system, while napD and napC are identified adjacent to the napAB genes of Alcaligenes eutrophus H16.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebersold R. H., Leavitt J., Saavedra R. A., Hood L. E., Kent S. B. Internal amino acid sequence analysis of proteins separated by one- or two-dimensional gel electrophoresis after in situ protease digestion on nitrocellulose. Proc Natl Acad Sci U S A. 1987 Oct;84(20):6970–6974. doi: 10.1073/pnas.84.20.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alef K., Klemme J. H. Assimilatory nitrate reductase of Rhodopseudomonas capsulata AD2: a molybdo-hemeprotein. Z Naturforsch C. 1979 Jan-Feb;34(1-2):33–37. doi: 10.1515/znc-1979-1-210. [DOI] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Axley M. J., Böck A., Stadtman T. C. Catalytic properties of an Escherichia coli formate dehydrogenase mutant in which sulfur replaces selenium. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8450–8454. doi: 10.1073/pnas.88.19.8450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axley M. J., Grahame D. A., Stadtman T. C. Escherichia coli formate-hydrogen lyase. Purification and properties of the selenium-dependent formate dehydrogenase component. J Biol Chem. 1990 Oct 25;265(30):18213–18218. [PubMed] [Google Scholar]
- Bell L. C., Page M. D., Berks B. C., Richardson D. J., Ferguson S. J. Insertion of transposon Tn5 into a structural gene of the membrane-bound nitrate reductase of Thiosphaera pantotropha results in anaerobic overexpression of periplasmic nitrate reductase activity. J Gen Microbiol. 1993 Dec;139(12):3205–3214. doi: 10.1099/00221287-139-12-3205. [DOI] [PubMed] [Google Scholar]
- Bell L. C., Richardson D. J., Ferguson S. J. Periplasmic and membrane-bound respiratory nitrate reductases in Thiosphaera pantotropha. The periplasmic enzyme catalyzes the first step in aerobic denitrification. FEBS Lett. 1990 Jun 4;265(1-2):85–87. doi: 10.1016/0014-5793(90)80889-q. [DOI] [PubMed] [Google Scholar]
- Bennett B., Benson N., McEwan A. G., Bray R. C. Multiple states of the molybdenum centre of dimethylsulphoxide reductase from Rhodobacter capsulatus revealed by EPR spectroscopy. Eur J Biochem. 1994 Oct 1;225(1):321–331. doi: 10.1111/j.1432-1033.1994.00321.x. [DOI] [PubMed] [Google Scholar]
- Benson N., Farrar J. A., McEwan A. G., Thomson A. J. Detection of the optical bands of molybdenum(V) in DMSO reductase (Rhodobacter capsulatus) by low-temperature MCD spectroscopy. FEBS Lett. 1992 Jul 28;307(2):169–172. doi: 10.1016/0014-5793(92)80760-e. [DOI] [PubMed] [Google Scholar]
- Berg B. L., Li J., Heider J., Stewart V. Nitrate-inducible formate dehydrogenase in Escherichia coli K-12. I. Nucleotide sequence of the fdnGHI operon and evidence that opal (UGA) encodes selenocysteine. J Biol Chem. 1991 Nov 25;266(33):22380–22385. [PubMed] [Google Scholar]
- Bergmann D. J., Arciero D. M., Hooper A. B. Organization of the hao gene cluster of Nitrosomonas europaea: genes for two tetraheme c cytochromes. J Bacteriol. 1994 Jun;176(11):3148–3153. doi: 10.1128/jb.176.11.3148-3153.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berks B. C., Richardson D. J., Robinson C., Reilly A., Aplin R. T., Ferguson S. J. Purification and characterization of the periplasmic nitrate reductase from Thiosphaera pantotropha. Eur J Biochem. 1994 Feb 15;220(1):117–124. doi: 10.1111/j.1432-1033.1994.tb18605.x. [DOI] [PubMed] [Google Scholar]
- Bilous P. T., Cole S. T., Anderson W. F., Weiner J. H. Nucleotide sequence of the dmsABC operon encoding the anaerobic dimethylsulphoxide reductase of Escherichia coli. Mol Microbiol. 1988 Nov;2(6):785–795. doi: 10.1111/j.1365-2958.1988.tb00090.x. [DOI] [PubMed] [Google Scholar]
- Blasco F., Iobbi C., Giordano G., Chippaux M., Bonnefoy V. Nitrate reductase of Escherichia coli: completion of the nucleotide sequence of the nar operon and reassessment of the role of the alpha and beta subunits in iron binding and electron transfer. Mol Gen Genet. 1989 Aug;218(2):249–256. doi: 10.1007/BF00331275. [DOI] [PubMed] [Google Scholar]
- Blasco F., Iobbi C., Ratouchniak J., Bonnefoy V., Chippaux M. Nitrate reductases of Escherichia coli: sequence of the second nitrate reductase and comparison with that encoded by the narGHJI operon. Mol Gen Genet. 1990 Jun;222(1):104–111. doi: 10.1007/BF00283030. [DOI] [PubMed] [Google Scholar]
- Blattner F. R., Burland V., Plunkett G., 3rd, Sofia H. J., Daniels D. L. Analysis of the Escherichia coli genome. IV. DNA sequence of the region from 89.2 to 92.8 minutes. Nucleic Acids Res. 1993 Nov 25;21(23):5408–5417. doi: 10.1093/nar/21.23.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokranz M., Gutmann M., Körtner C., Kojro E., Fahrenholz F., Lauterbach F., Kröger A. Cloning and nucleotide sequence of the structural genes encoding the formate dehydrogenase of Wolinella succinogenes. Arch Microbiol. 1991;156(2):119–128. doi: 10.1007/BF00290984. [DOI] [PubMed] [Google Scholar]
- Breton J., Berks B. C., Reilly A., Thomson A. J., Ferguson S. J., Richardson D. J. Characterization of the paramagnetic iron-containing redox centres of Thiosphaera pantotropha periplasmic nitrate reductase. FEBS Lett. 1994 May 23;345(1):76–80. doi: 10.1016/0014-5793(94)00445-5. [DOI] [PubMed] [Google Scholar]
- Bursakov S., Liu M. Y., Payne W. J., LeGall J., Moura I., Moura J. J. Isolation and preliminary characterization of a soluble nitrate reductase from the sulfate reducing organism Desulfovibrio desulfuricans ATCC 27774. Anaerobe. 1995 Feb;1(1):55–60. doi: 10.1016/s1075-9964(95)80444-7. [DOI] [PubMed] [Google Scholar]
- Chistoserdov A. Y., Chistoserdova L. V., McIntire W. S., Lidstrom M. E. Genetic organization of the mau gene cluster in Methylobacterium extorquens AM1: complete nucleotide sequence and generation and characteristics of mau mutants. J Bacteriol. 1994 Jul;176(13):4052–4065. doi: 10.1128/jb.176.13.4052-4065.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chistoserdov A. Y., McIntire W. S., Mathews F. S., Lidstrom M. E. Organization of the methylamine utilization (mau) genes in Methylophilus methylotrophus W3A1-NS. J Bacteriol. 1994 Jul;176(13):4073–4080. doi: 10.1128/jb.176.13.4073-4080.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe M., Reznikoff W. S. Anaerobically expressed Escherichia coli genes identified by operon fusion techniques. J Bacteriol. 1991 Oct;173(19):6139–6146. doi: 10.1128/jb.173.19.6139-6146.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe M., Reznikoff W. S. Identification of the regulatory sequence of anaerobically expressed locus aeg-46.5. J Bacteriol. 1993 Feb;175(4):1165–1172. doi: 10.1128/jb.175.4.1165-1172.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolata M. M., Van Beeumen J. J., Ambler R. P., Meyer T. E., Cusanovich M. A. Nucleotide sequence of the heme subunit of flavocytochrome c from the purple phototrophic bacterium, Chromatium vinosum. A 2.6-kilobase pair DNA fragment contains two multiheme cytochromes, a flavoprotein, and a homolog of human ankyrin. J Biol Chem. 1993 Jul 5;268(19):14426–14431. [PubMed] [Google Scholar]
- Engelman D. M., Steitz T. A., Goldman A. Identifying nonpolar transbilayer helices in amino acid sequences of membrane proteins. Annu Rev Biophys Biophys Chem. 1986;15:321–353. doi: 10.1146/annurev.bb.15.060186.001541. [DOI] [PubMed] [Google Scholar]
- Friedebold J., Bowien B. Physiological and biochemical characterization of the soluble formate dehydrogenase, a molybdoenzyme from Alcaligenes eutrophus. J Bacteriol. 1993 Aug;175(15):4719–4728. doi: 10.1128/jb.175.15.4719-4728.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladyshev V. N., Khangulov S. V., Axley M. J., Stadtman T. C. Coordination of selenium to molybdenum in formate dehydrogenase H from Escherichia coli. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7708–7711. doi: 10.1073/pnas.91.16.7708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heider J., Böck A. Selenium metabolism in micro-organisms. Adv Microb Physiol. 1993;35:71–109. doi: 10.1016/s0065-2911(08)60097-1. [DOI] [PubMed] [Google Scholar]
- Hille R. The reaction mechanism of oxomolybdenum enzymes. Biochim Biophys Acta. 1994 Mar 8;1184(2-3):143–169. doi: 10.1016/0005-2728(94)90220-8. [DOI] [PubMed] [Google Scholar]
- Iobbi-Nivol C., Crooke H., Griffiths L., Grove J., Hussain H., Pommier J., Mejean V., Cole J. A. A reassessment of the range of c-type cytochromes synthesized by Escherichia coli K-12. FEMS Microbiol Lett. 1994 Jun 1;119(1-2):89–94. doi: 10.1111/j.1574-6968.1994.tb06872.x. [DOI] [PubMed] [Google Scholar]
- Jones D. T., Taylor W. R., Thornton J. M. A model recognition approach to the prediction of all-helical membrane protein structure and topology. Biochemistry. 1994 Mar 15;33(10):3038–3049. doi: 10.1021/bi00176a037. [DOI] [PubMed] [Google Scholar]
- Jüngst A., Wakabayashi S., Matsubara H., Zumft W. G. The nirSTBM region coding for cytochrome cd1-dependent nitrite respiration of Pseudomonas stutzeri consists of a cluster of mono-, di-, and tetraheme proteins. FEBS Lett. 1991 Feb 25;279(2):205–209. doi: 10.1016/0014-5793(91)80150-2. [DOI] [PubMed] [Google Scholar]
- Karzanov V. V., Bogatsky YuA, Tishkov V. I., Egorov A. M. Evidence for the presence of a new NAD+-dependent formate dehydrogenase in Pseudomonas sp. 101 cells grown on a molybdenum-containing medium. FEMS Microbiol Lett. 1989 Jul 15;51(1):197–200. doi: 10.1016/0378-1097(89)90508-9. [DOI] [PubMed] [Google Scholar]
- Kilpatrick L., Rajagopalan K. V., Hilton J., Bastian N. R., Stiefel E. I., Pilato R. S., Spiro T. G. Resonance Raman spectroscopic characterization of the molybdopterin active site of DMSO reductase. Biochemistry. 1995 Mar 7;34(9):3032–3039. doi: 10.1021/bi00009a034. [DOI] [PubMed] [Google Scholar]
- Krafft T., Bokranz M., Klimmek O., Schröder I., Fahrenholz F., Kojro E., Kröger A. Cloning and nucleotide sequence of the psrA gene of Wolinella succinogenes polysulphide reductase. Eur J Biochem. 1992 Jun 1;206(2):503–510. doi: 10.1111/j.1432-1033.1992.tb16953.x. [DOI] [PubMed] [Google Scholar]
- Krishtalik L. I., Tae G. S., Cherepanov D. A., Cramer W. A. The redox properties of cytochromes b imposed by the membrane electrostatic environment. Biophys J. 1993 Jul;65(1):184–195. doi: 10.1016/S0006-3495(93)81050-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kröger A., Winkler E., Innerhofer A., Hackenberg H., Schägger H. The formate dehydrogenase involved in electron transport from formate to fumarate in Vibrio succinogenes. Eur J Biochem. 1979 Mar;94(2):465–475. doi: 10.1111/j.1432-1033.1979.tb12914.x. [DOI] [PubMed] [Google Scholar]
- Lin J. T., Goldman B. S., Stewart V. Structures of genes nasA and nasB, encoding assimilatory nitrate and nitrite reductases in Klebsiella pneumoniae M5al. J Bacteriol. 1993 Apr;175(8):2370–2378. doi: 10.1128/jb.175.8.2370-2378.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig W., Mittenhuber G., Friedrich C. G. Transfer of Thiosphaera pantotropha to Paracoccus denitrificans. Int J Syst Bacteriol. 1993 Apr;43(2):363–367. doi: 10.1099/00207713-43-2-363. [DOI] [PubMed] [Google Scholar]
- Lübben M., Warne A., Albracht S. P., Saraste M. The purified SoxABCD quinol oxidase complex of Sulfolobus acidocaldarius contains a novel haem. Mol Microbiol. 1994 Jul;13(2):327–335. doi: 10.1111/j.1365-2958.1994.tb00426.x. [DOI] [PubMed] [Google Scholar]
- Maskell D. J., Szabo M. J., Butler P. D., Williams A. E., Moxon E. R. Molecular analysis of a complex locus from Haemophilus influenzae involved in phase-variable lipopolysaccharide biosynthesis. Mol Microbiol. 1991 May;5(5):1013–1022. doi: 10.1111/j.1365-2958.1991.tb01874.x. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Méjean V., Iobbi-Nivol C., Lepelletier M., Giordano G., Chippaux M., Pascal M. C. TMAO anaerobic respiration in Escherichia coli: involvement of the tor operon. Mol Microbiol. 1994 Mar;11(6):1169–1179. doi: 10.1111/j.1365-2958.1994.tb00393.x. [DOI] [PubMed] [Google Scholar]
- Ogasawara N., Nakai S., Yoshikawa H. Systematic sequencing of the 180 kilobase region of the Bacillus subtilis chromosome containing the replication origin. DNA Res. 1994;1(1):1–14. doi: 10.1093/dnares/1.1.1. [DOI] [PubMed] [Google Scholar]
- Persson B., Argos P. Prediction of transmembrane segments in proteins utilising multiple sequence alignments. J Mol Biol. 1994 Mar 25;237(2):182–192. doi: 10.1006/jmbi.1994.1220. [DOI] [PubMed] [Google Scholar]
- Phillips J. D., Graham L. A., Trumpower B. L. Subunit 9 of the Saccharomyces cerevisiae cytochrome bc1 complex is required for insertion of EPR-detectable iron-sulfur cluster into the Rieske iron-sulfur protein. J Biol Chem. 1993 Jun 5;268(16):11727–11736. [PubMed] [Google Scholar]
- Rabin R. S., Stewart V. Dual response regulators (NarL and NarP) interact with dual sensors (NarX and NarQ) to control nitrate- and nitrite-regulated gene expression in Escherichia coli K-12. J Bacteriol. 1993 Jun;175(11):3259–3268. doi: 10.1128/jb.175.11.3259-3268.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan K. V., Johnson J. L. The pterin molybdenum cofactors. J Biol Chem. 1992 May 25;267(15):10199–10202. [PubMed] [Google Scholar]
- Richardson D. J., McEwan A. G., Page M. D., Jackson J. B., Ferguson S. J. The identification of cytochromes involved in the transfer of electrons to the periplasmic NO3- reductase of Rhodobacter capsulatus and resolution of a soluble NO3(-)-reductase--cytochrome-c552 redox complex. Eur J Biochem. 1990 Nov 26;194(1):263–270. doi: 10.1111/j.1432-1033.1990.tb19452.x. [DOI] [PubMed] [Google Scholar]
- Rossi M., Pollock W. B., Reij M. W., Keon R. G., Fu R., Voordouw G. The hmc operon of Desulfovibrio vulgaris subsp. vulgaris Hildenborough encodes a potential transmembrane redox protein complex. J Bacteriol. 1993 Aug;175(15):4699–4711. doi: 10.1128/jb.175.15.4699-4711.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samyn B., Berks B. C., Page M. D., Ferguson S. J., van Beeumen J. J. Characterisation and amino acid sequence of cytochrome c-550 from Thiosphaera pantotropha. Eur J Biochem. 1994 Jan 15;219(1-2):585–594. doi: 10.1111/j.1432-1033.1994.tb19974.x. [DOI] [PubMed] [Google Scholar]
- Schauer N. L., Ferry J. G. Composition of the coenzyme F420-dependent formate dehydrogenase from Methanobacterium formicicum. J Bacteriol. 1986 Feb;165(2):405–411. doi: 10.1128/jb.165.2.405-411.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuber A. P., Orr E. C., Recny M. A., Schendel P. F., May H. D., Schauer N. L., Ferry J. G. Cloning, expression, and nucleotide sequence of the formate dehydrogenase genes from Methanobacterium formicicum. J Biol Chem. 1986 Oct 5;261(28):12942–12947. [PubMed] [Google Scholar]
- Siddiqui R. A., Warnecke-Eberz U., Hengsberger A., Schneider B., Kostka S., Friedrich B. Structure and function of a periplasmic nitrate reductase in Alcaligenes eutrophus H16. J Bacteriol. 1993 Sep;175(18):5867–5876. doi: 10.1128/jb.175.18.5867-5876.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. B., Tiedje J. M. Isolation and characterization of a nitrite reductase gene and its use as a probe for denitrifying bacteria. Appl Environ Microbiol. 1992 Jan;58(1):376–384. doi: 10.1128/aem.58.1.376-384.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofia H. J., Burland V., Daniels D. L., Plunkett G., 3rd, Blattner F. R. Analysis of the Escherichia coli genome. V. DNA sequence of the region from 76.0 to 81.5 minutes. Nucleic Acids Res. 1994 Jul 11;22(13):2576–2586. doi: 10.1093/nar/22.13.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trieber C. A., Rothery R. A., Weiner J. H. Multiple pathways of electron transfer in dimethyl sulfoxide reductase of Escherichia coli. J Biol Chem. 1994 Mar 11;269(10):7103–7109. [PubMed] [Google Scholar]
- Walker J. E., Arizmendi J. M., Dupuis A., Fearnley I. M., Finel M., Medd S. M., Pilkington S. J., Runswick M. J., Skehel J. M. Sequences of 20 subunits of NADH:ubiquinone oxidoreductase from bovine heart mitochondria. Application of a novel strategy for sequencing proteins using the polymerase chain reaction. J Mol Biol. 1992 Aug 20;226(4):1051–1072. doi: 10.1016/0022-2836(92)91052-q. [DOI] [PubMed] [Google Scholar]
- Wood P. M. Why do c-type cytochromes exist? FEBS Lett. 1983 Dec 12;164(2):223–226. doi: 10.1016/0014-5793(83)80289-0. [DOI] [PubMed] [Google Scholar]
- Wootton J. C., Nicolson R. E., Cock J. M., Walters D. E., Burke J. F., Doyle W. A., Bray R. C. Enzymes depending on the pterin molybdenum cofactor: sequence families, spectroscopic properties of molybdenum and possible cofactor-binding domains. Biochim Biophys Acta. 1991 Mar 29;1057(2):157–185. doi: 10.1016/s0005-2728(05)80100-8. [DOI] [PubMed] [Google Scholar]
- Ye R. W., Fries M. R., Bezborodnikov S. G., Averill B. A., Tiedje J. M. Characterization of the structural gene encoding a copper-containing nitrite reductase and homology of this gene to DNA of other denitrifiers. Appl Environ Microbiol. 1993 Jan;59(1):250–254. doi: 10.1128/aem.59.1.250-254.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinoni F., Birkmann A., Stadtman T. C., Böck A. Nucleotide sequence and expression of the selenocysteine-containing polypeptide of formate dehydrogenase (formate-hydrogen-lyase-linked) from Escherichia coli. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4650–4654. doi: 10.1073/pnas.83.13.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Membrane protein structure prediction. Hydrophobicity analysis and the positive-inside rule. J Mol Biol. 1992 May 20;225(2):487–494. doi: 10.1016/0022-2836(92)90934-c. [DOI] [PubMed] [Google Scholar]


