Abstract
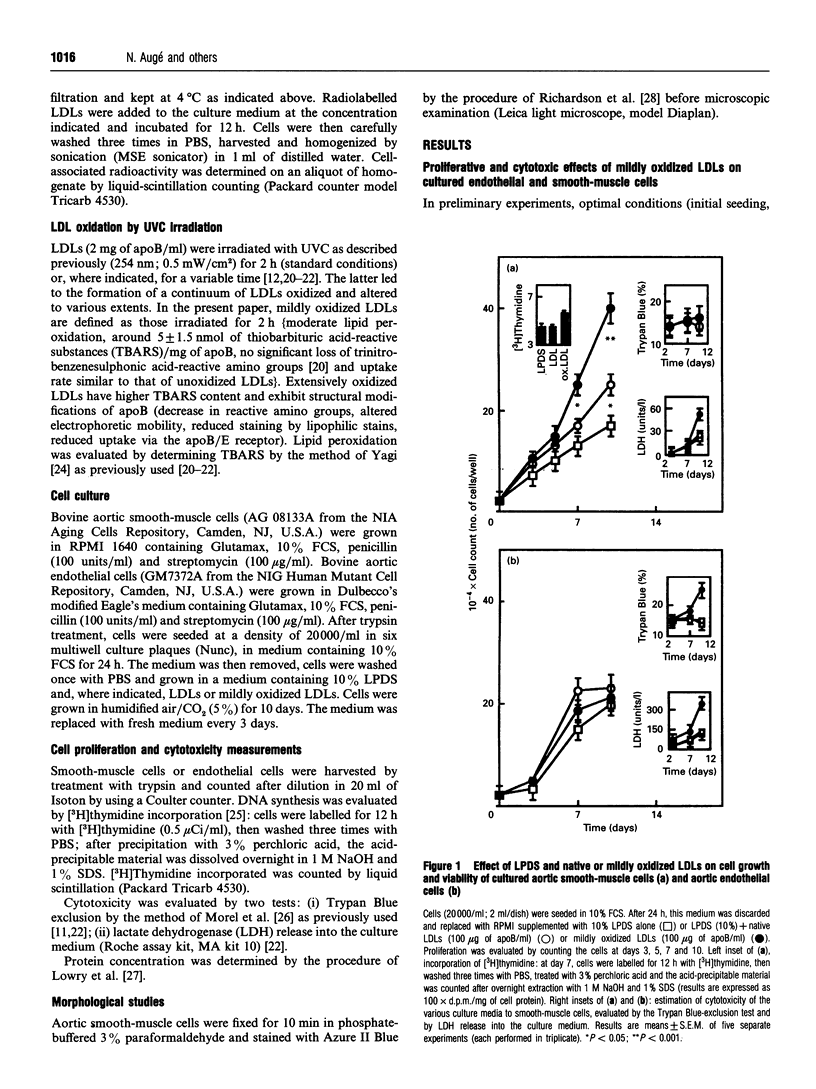
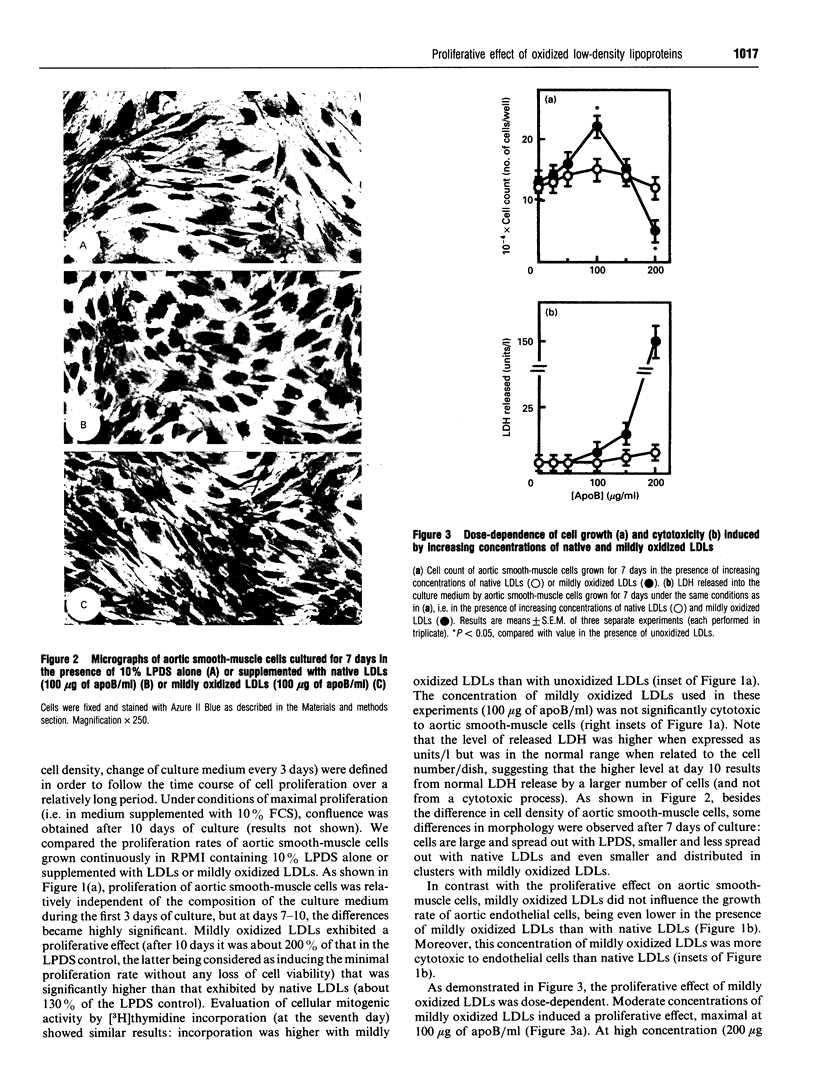
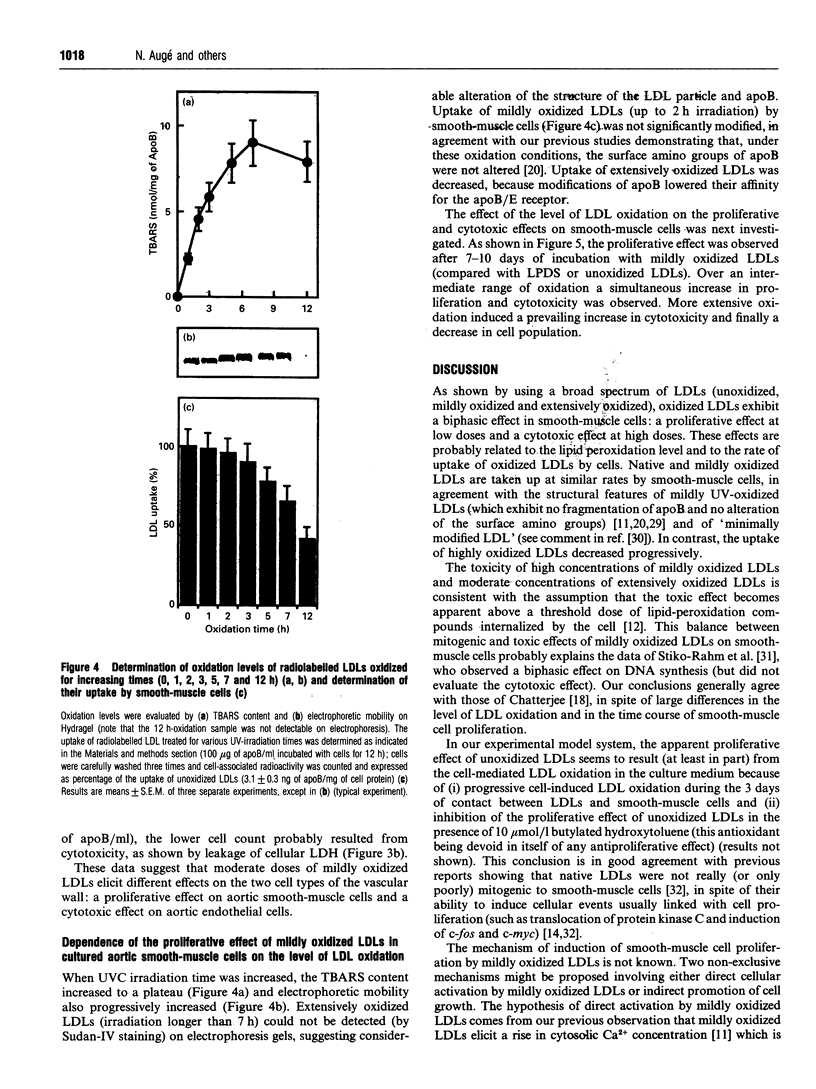
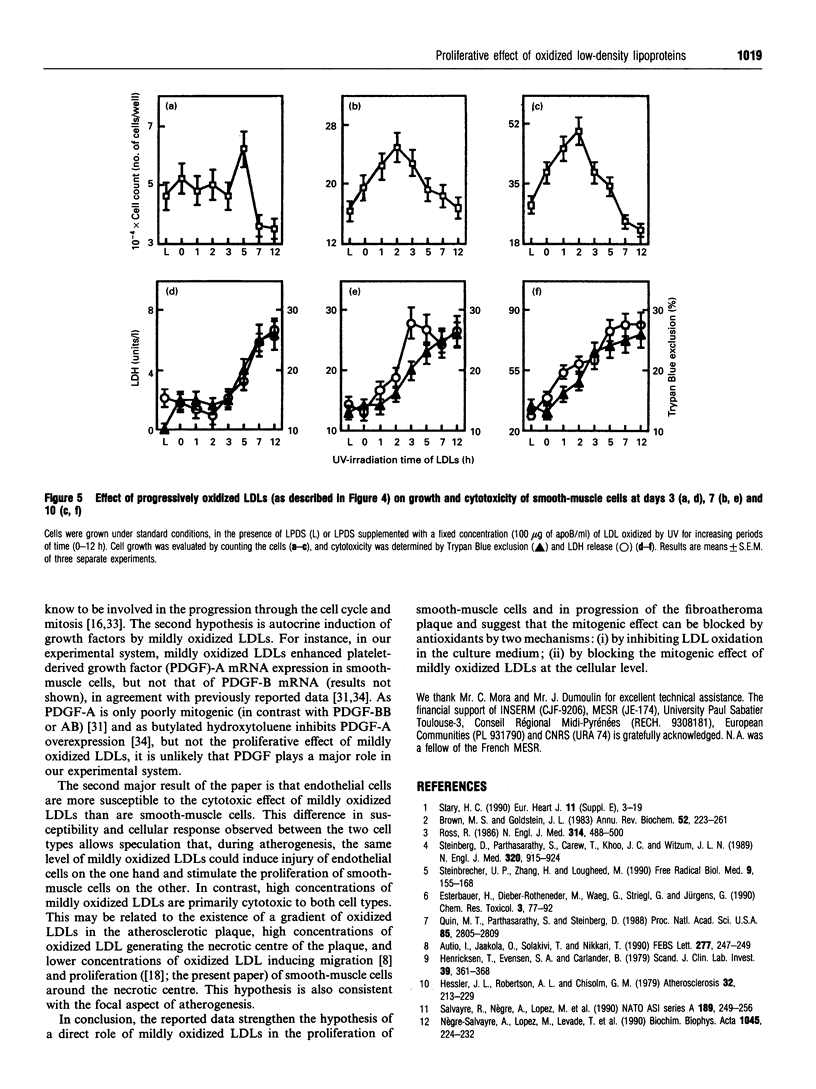
We have investigated the role of low-density lipoprotein (LDL) oxidation in the proliferative effect of LDLs on cultured bovine aortic smooth-muscle cells and compared it with their effect on bovine aortic endothelial cells. The following conclusions were reached. (1) Non-toxic doses of mildly oxidized LDLs elicit a proliferative effect on smooth-muscle cells significantly higher than that of native LDLs or lipoprotein-depleted serum. The proliferative effect is dependent on time (relatively slow), dose (high doses are cytotoxic) and the level of LDL oxidation. (2) The proliferative effect on smooth-muscle cells is counterbalanced at high concentrations of mildly oxidized LDLs (or at high oxidation levels) by their cytotoxic effect. (3) The same dose of mildly oxidized LDLs exhibits no proliferative effect on endothelial cells but rather a cytotoxic one. Endothelial cells may therefore be intrinsically more susceptible to the cytotoxic effect of mildly oxidized LDLs than are smooth-muscle cells. (4) The proliferative effect of native LDLs on smooth-muscle cells results (at least in part) from cell-induced LDL oxidation during cell culture as suggested by (i) the progressive LDL oxidation over the 3 days of contact between LDLs and smooth-muscle cells and (ii) the concomitant inhibition of LDL oxidation and proliferative effect by butylated hydroxytoluene. The hypothetical mechanisms and potential involvement in atherogenesis are discussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Autio I., Jaakkola O., Solakivi T., Nikkari T. Oxidized low-density lipoprotein is chemotactic for arterial smooth muscle cells in culture. FEBS Lett. 1990 Dec 17;277(1-2):247–249. doi: 10.1016/0014-5793(90)80857-f. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem. 1983;52:223–261. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- Chatterjee S. Role of oxidized human plasma low density lipoproteins in atherosclerosis: effects on smooth muscle cell proliferation. Mol Cell Biochem. 1992 Apr;111(1-2):143–147. doi: 10.1007/BF00229586. [DOI] [PubMed] [Google Scholar]
- Dousset N., Negre-Salvayre A., Lopez M., Salvayre R., Douste-Blazy L. Ultraviolet-treated lipoproteins as a model system for the study of the biological effects of lipid peroxides on cultured cell. I. Chemical modifications of ultraviolet-treated low-density lipoproteins. Biochim Biophys Acta. 1990 Aug 6;1045(3):219–223. doi: 10.1016/0005-2760(90)90123-f. [DOI] [PubMed] [Google Scholar]
- Esterbauer H., Dieber-Rotheneder M., Waeg G., Striegl G., Jürgens G. Biochemical, structural, and functional properties of oxidized low-density lipoprotein. Chem Res Toxicol. 1990 Mar-Apr;3(2):77–92. doi: 10.1021/tx00014a001. [DOI] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Hooker S., Sanford G. L., Montgomery V., Rivers R., Emmett N. Influence of low density lipoproteins on vascular smooth muscle cell growth and motility: modulation by extracellular matrix. Cell Biol Int Rep. 1992 May;16(5):433–450. doi: 10.1016/s0309-1651(06)80063-9. [DOI] [PubMed] [Google Scholar]
- Henriksen T., Evensen S. A., Carlander B. Injury to human endothelial cells in culture induced by low density lipoproteins. Scand J Clin Lab Invest. 1979 Jun;39(4):361–368. doi: 10.3109/00365517909106120. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lu K. P., Means A. R. Regulation of the cell cycle by calcium and calmodulin. Endocr Rev. 1993 Feb;14(1):40–58. doi: 10.1210/edrv-14-1-40. [DOI] [PubMed] [Google Scholar]
- Morel D. W., DiCorleto P. E., Chisolm G. M. Endothelial and smooth muscle cells alter low density lipoprotein in vitro by free radical oxidation. Arteriosclerosis. 1984 Jul-Aug;4(4):357–364. doi: 10.1161/01.atv.4.4.357. [DOI] [PubMed] [Google Scholar]
- Negre-Salvayre A., Lopez M., Levade T., Pieraggi M. T., Dousset N., Douste-Blazy L., Salvayre R. Ultraviolet-treated lipoproteins as a model system for the study of the biological effects of lipid peroxides on cultured cells. II. Uptake and cytotoxicity of ultraviolet-treated LDL on lymphoid cell lines. Biochim Biophys Acta. 1990 Aug 6;1045(3):224–232. doi: 10.1016/0005-2760(90)90124-g. [DOI] [PubMed] [Google Scholar]
- Nègre-Salvayre A., Fitoussi G., Réaud V., Pieraggi M. T., Thiers J. C., Salvayre R. A delayed and sustained rise of cytosolic calcium is elicited by oxidized LDL in cultured bovine aortic endothelial cells. FEBS Lett. 1992 Mar 24;299(1):60–65. doi: 10.1016/0014-5793(92)80101-l. [DOI] [PubMed] [Google Scholar]
- Nègre-Salvayre A., Paillous N., Dousset N., Bascoul J., Salvayre R. Wavelength dependence of photoinduced peroxidation and cytotoxicity of human low density lipoproteins. Photochem Photobiol. 1992 Feb;55(2):197–204. doi: 10.1111/j.1751-1097.1992.tb04228.x. [DOI] [PubMed] [Google Scholar]
- Nègre-Salvayre A., Salvayre R. Protection by Ca2+ channel blockers (nifedipine, diltiazem and verapamil) against the toxicity of oxidized low density lipoprotein to cultured lymphoid cells. Br J Pharmacol. 1992 Nov;107(3):738–744. doi: 10.1111/j.1476-5381.1992.tb14516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nègre-Salvayre A., Salvayre R. Quercetin prevents the cytotoxicity of oxidized LDL on lymphoid cell lines. Free Radic Biol Med. 1992;12(2):101–106. doi: 10.1016/0891-5849(92)90002-x. [DOI] [PubMed] [Google Scholar]
- Ozer N. K., Palozza P., Boscoboinik D., Azzi A. d-alpha-Tocopherol inhibits low density lipoprotein induced proliferation and protein kinase C activity in vascular smooth muscle cells. FEBS Lett. 1993 May 17;322(3):307–310. doi: 10.1016/0014-5793(93)81592-n. [DOI] [PubMed] [Google Scholar]
- Quinn M. T., Parthasarathy S., Steinberg D. Lysophosphatidylcholine: a chemotactic factor for human monocytes and its potential role in atherogenesis. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2805–2809. doi: 10.1073/pnas.85.8.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDSON K. C., JARETT L., FINKE E. H. Embedding in epoxy resins for ultrathin sectioning in electron microscopy. Stain Technol. 1960 Nov;35:313–323. doi: 10.3109/10520296009114754. [DOI] [PubMed] [Google Scholar]
- Rajavashisth T. B., Andalibi A., Territo M. C., Berliner J. A., Navab M., Fogelman A. M., Lusis A. J. Induction of endothelial cell expression of granulocyte and macrophage colony-stimulating factors by modified low-density lipoproteins. Nature. 1990 Mar 15;344(6263):254–257. doi: 10.1038/344254a0. [DOI] [PubMed] [Google Scholar]
- Roberts D. C., Miller N. E., Price S. G., Crook D., Cortese C., La Ville A., Masana L., Lewis B. An alternative procedure for incorporating radiolabelled cholesteryl ester into human plasma lipoproteins in vitro. Biochem J. 1985 Feb 15;226(1):319–322. doi: 10.1042/bj2260319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Scott-Burden T., Resink T. J., Hahn A. P., Bühler F. R. Differential stimulation of growth related metabolism in cultured smooth muscle cells from SHR and WKY rats by combinations of EGF and LDL. Biochem Biophys Res Commun. 1989 Mar 15;159(2):624–632. doi: 10.1016/0006-291x(89)90040-5. [DOI] [PubMed] [Google Scholar]
- Scott-Burden T., Resink T. J., Hahn A. W., Baur U., Box R. J., Bühler F. R. Induction of growth-related metabolism in human vascular smooth muscle cells by low density lipoprotein. J Biol Chem. 1989 Jul 25;264(21):12582–12589. [PubMed] [Google Scholar]
- Stary H. C. The sequence of cell and matrix changes in atherosclerotic lesions of coronary arteries in the first forty years of life. Eur Heart J. 1990 Aug;11 (Suppl E):3–19. doi: 10.1093/eurheartj/11.suppl_e.3. [DOI] [PubMed] [Google Scholar]
- Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., Witztum J. L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989 Apr 6;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Steinbrecher U. P., Zhang H. F., Lougheed M. Role of oxidatively modified LDL in atherosclerosis. Free Radic Biol Med. 1990;9(2):155–168. doi: 10.1016/0891-5849(90)90119-4. [DOI] [PubMed] [Google Scholar]
- Stiko-Rahm A., Hultgårdh-Nilsson A., Regnström J., Hamsten A., Nilsson J. Native and oxidized LDL enhances production of PDGF AA and the surface expression of PDGF receptors in cultured human smooth muscle cells. Arterioscler Thromb. 1992 Sep;12(9):1099–1109. doi: 10.1161/01.atv.12.9.1099. [DOI] [PubMed] [Google Scholar]
- Whitaker M., Patel R. Calcium and cell cycle control. Development. 1990 Apr;108(4):525–542. doi: 10.1242/dev.108.4.525. [DOI] [PubMed] [Google Scholar]
- Yagi K. Lipid peroxides and human diseases. Chem Phys Lipids. 1987 Nov-Dec;45(2-4):337–351. doi: 10.1016/0009-3084(87)90071-5. [DOI] [PubMed] [Google Scholar]
- Zwijsen R. M., Japenga S. C., Heijen A. M., van den Bos R. C., Koeman J. H. Induction of platelet-derived growth factor chain A gene expression in human smooth muscle cells by oxidized low density lipoproteins. Biochem Biophys Res Commun. 1992 Aug 14;186(3):1410–1416. doi: 10.1016/s0006-291x(05)81563-3. [DOI] [PubMed] [Google Scholar]



