Abstract
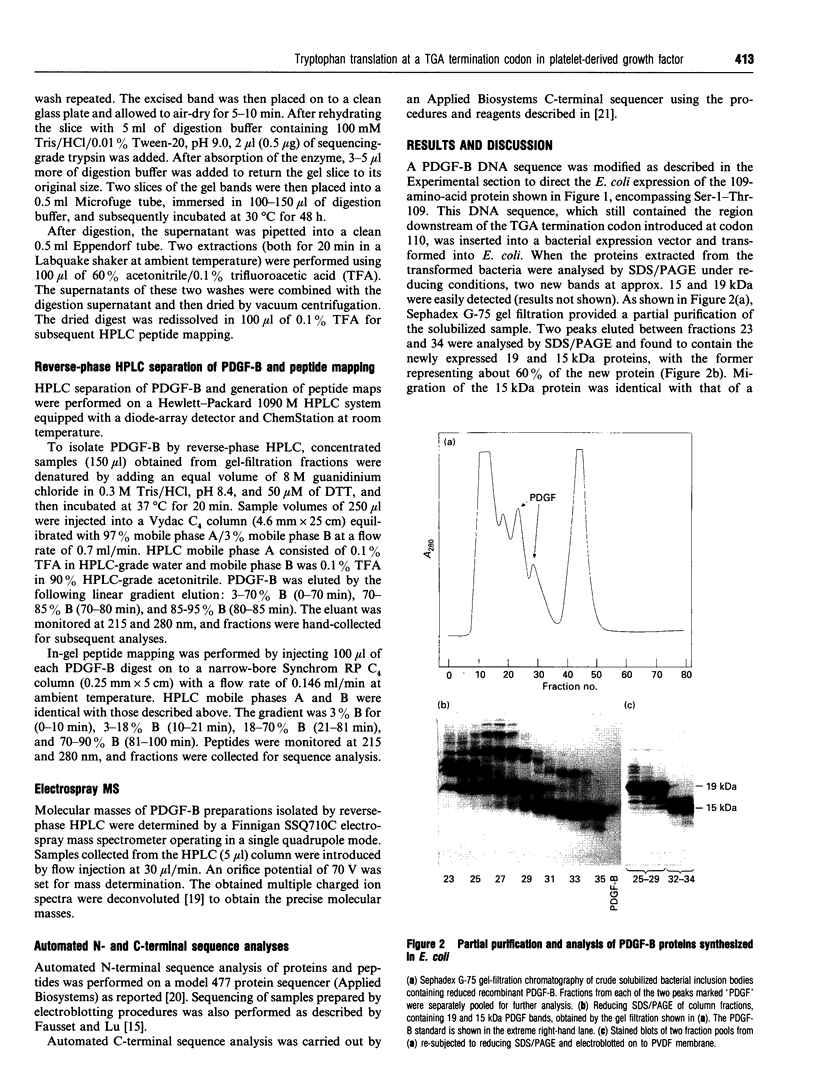
The mature 109-amino-acid human platelet-derived growth factor B (PDGF-B) peptide is derived by intracellular processing from a 241-amino-acid precursor synthesized in mammalian cells, with removal of 81 N-terminal and 51 C-terminal amino acids. In order to produce directly the mature 109-amino acid PDGF-B peptide as a recombinant protein in Escherichia coli, a CGA codon at position 110 of a DNA sequence encoding the full-length precursor form of PDGF-B was converted into the translation termination codon TGA by in vitro mutagenesis. Expression of this DNA via a plasmid vector in E. coli resulted in production of two distinct PDGF-B proteins having apparent molecular masses of 15 and 19 kDa, with the latter species predominating. Structural characterization employing N- and C-terminal amino acid sequencing and MS analyses indicated that the 15 kDa protein is the expected 109-amino-acid PDGF-B, and that the 19 kDa protein represents a C-terminal extended PDGF-B containing 160 amino acids. Characterization of a unique tryptic peptide derived from the 19 kDa protein revealed that this longer form of PDGF-B results from mistranslation of the introduced TGA termination codon at position 110 as tryptophan, with translation subsequently proceeding to the naturally occurring TAG termination codon at position 161. Owing to the high rate of translation readthrough of TGA codons in this and occasionally other proteins, it appears that the use of TGA as a translation termination codon for proteins to be expressed in E. coli should be avoided when possible.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boone T., Chazin V., Kenney W., Swanson E., Altrock B. Construction, purification and biological activities of recombinant human interleukin-2 analogs. Dev Biol Stand. 1988;69:157–168. [PubMed] [Google Scholar]
- Bouadloun F., Donner D., Kurland C. G. Codon-specific missense errors in vivo. EMBO J. 1983;2(8):1351–1356. doi: 10.1002/j.1460-2075.1983.tb01591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd V. L., Bozzini M., Zon G., Noble R. L., Mattaliano R. J. Sequencing of peptides and proteins from the carboxy terminus. Anal Biochem. 1992 Nov 1;206(2):344–352. doi: 10.1016/0003-2697(92)90376-i. [DOI] [PubMed] [Google Scholar]
- Clements J. M., Bawden L. J., Bloxidge R. E., Catlin G., Cook A. L., Craig S., Drummond A. H., Edwards R. M., Fallon A., Green D. R. Two PDGF-B chain residues, arginine 27 and isoleucine 30, mediate receptor binding and activation. EMBO J. 1991 Dec;10(13):4113–4120. doi: 10.1002/j.1460-2075.1991.tb04988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craigen W. J., Lee C. C., Caskey C. T. Recent advances in peptide chain termination. Mol Microbiol. 1990 Jun;4(6):861–865. doi: 10.1111/j.1365-2958.1990.tb00658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggertsson G., Söll D. Transfer ribonucleic acid-mediated suppression of termination codons in Escherichia coli. Microbiol Rev. 1988 Sep;52(3):354–374. doi: 10.1128/mr.52.3.354-374.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausset P. R., Lu H. S. Structural analysis of recombinant proteins prepared by semi-dry electroblotting after sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Electrophoresis. 1991 Jan;12(1):22–27. doi: 10.1002/elps.1150120106. [DOI] [PubMed] [Google Scholar]
- Fox T. D. Natural variation in the genetic code. Annu Rev Genet. 1987;21:67–91. doi: 10.1146/annurev.ge.21.120187.000435. [DOI] [PubMed] [Google Scholar]
- Gould G. W., Lienhard G. E., Tanner L. I., Gibbs E. M. Phenylarsine oxide stimulates hexose transport in 3T3-L1 adipocytes by a mechanism other than an increase in surface transporters. Arch Biochem Biophys. 1989 Jan;268(1):264–275. doi: 10.1016/0003-9861(89)90588-2. [DOI] [PubMed] [Google Scholar]
- Heldin C. H. Structural and functional studies on platelet-derived growth factor. EMBO J. 1992 Dec;11(12):4251–4259. doi: 10.1002/j.1460-2075.1992.tb05523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsson A., Heldin C. H., Wasteson A., Westermark B., Deuel T. F., Huang J. S., Seeburg P. H., Gray A., Ullrich A., Scrace G. The c-sis gene encodes a precursor of the B chain of platelet-derived growth factor. EMBO J. 1984 May;3(5):921–928. doi: 10.1002/j.1460-2075.1984.tb01908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston T. C., Borgia P. T., Parker J. Codon specificity of starvation induced misreading. Mol Gen Genet. 1984;195(3):459–465. doi: 10.1007/BF00341447. [DOI] [PubMed] [Google Scholar]
- Kawasaki H., Emori Y., Suzuki K. Production and separation of peptides from proteins stained with Coomassie brilliant blue R-250 after separation by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Anal Biochem. 1990 Dec;191(2):332–336. doi: 10.1016/0003-2697(90)90227-z. [DOI] [PubMed] [Google Scholar]
- Kenney W. C., Haniu M., Herman A. C., Arakawa T., Costigan V. J., Lary J., Yphantis D. A., Thomason A. R. Formation of mitogenically active PDGF-B dimer does not require interchain disulfide bonds. J Biol Chem. 1994 Apr 22;269(16):12351–12359. [PubMed] [Google Scholar]
- Kopelowitz J., Hampe C., Goldman R., Reches M., Engelberg-Kulka H. Influence of codon context on UGA suppression and readthrough. J Mol Biol. 1992 May 20;225(2):261–269. doi: 10.1016/0022-2836(92)90920-f. [DOI] [PubMed] [Google Scholar]
- Kurland C. G., Ehrenberg M. Growth-optimizing accuracy of gene expression. Annu Rev Biophys Biophys Chem. 1987;16:291–317. doi: 10.1146/annurev.bb.16.060187.001451. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leinfelder W., Zehelein E., Mandrand-Berthelot M. A., Böck A. Gene for a novel tRNA species that accepts L-serine and cotranslationally inserts selenocysteine. Nature. 1988 Feb 25;331(6158):723–725. doi: 10.1038/331723a0. [DOI] [PubMed] [Google Scholar]
- Lu H. S., Fausset P. R., Sotos L. S., Clogston C. L., Rohde M. F., Stoney K. S., Herman A. C. Isolation and characterization of three recombinant human granulocyte colony stimulating factor His-->Gln isoforms produced in Escherichia coli. Protein Expr Purif. 1993 Oct;4(5):465–472. doi: 10.1006/prep.1993.1061. [DOI] [PubMed] [Google Scholar]
- Lu H. S., Tsai L. B., Kenney W. C., Lai P. H. Identification of unusual replacement of methionine by norleucine in recombinant interleukin-2 produced by E. coli. Biochem Biophys Res Commun. 1988 Oct 31;156(2):807–813. doi: 10.1016/s0006-291x(88)80916-1. [DOI] [PubMed] [Google Scholar]
- Oefner C., D'Arcy A., Winkler F. K., Eggimann B., Hosang M. Crystal structure of human platelet-derived growth factor BB. EMBO J. 1992 Nov;11(11):3921–3926. doi: 10.1002/j.1460-2075.1992.tb05485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker J. Errors and alternatives in reading the universal genetic code. Microbiol Rev. 1989 Sep;53(3):273–298. doi: 10.1128/mr.53.3.273-298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines E. W., Ross R. Compartmentalization of PDGF on extracellular binding sites dependent on exon-6-encoded sequences. J Cell Biol. 1992 Jan;116(2):533–543. doi: 10.1083/jcb.116.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld J., Capdevielle J., Guillemot J. C., Ferrara P. In-gel digestion of proteins for internal sequence analysis after one- or two-dimensional gel electrophoresis. Anal Biochem. 1992 May 15;203(1):173–179. doi: 10.1016/0003-2697(92)90061-b. [DOI] [PubMed] [Google Scholar]
- Santos M. A., Tuite M. F. New insights into mRNA decoding--implications for heterologous protein synthesis. Trends Biotechnol. 1993 Dec;11(12):500–505. doi: 10.1016/0167-7799(93)90028-8. [DOI] [PubMed] [Google Scholar]
- Seetharam R., Heeren R. A., Wong E. Y., Braford S. R., Klein B. K., Aykent S., Kotts C. E., Mathis K. J., Bishop B. F., Jennings M. J. Mistranslation in IGF-1 during over-expression of the protein in Escherichia coli using a synthetic gene containing low frequency codons. Biochem Biophys Res Commun. 1988 Aug 30;155(1):518–523. doi: 10.1016/s0006-291x(88)81117-3. [DOI] [PubMed] [Google Scholar]
- Söll D. Genetic code: enter a new amino acid. Nature. 1988 Feb 25;331(6158):662–663. doi: 10.1038/331662a0. [DOI] [PubMed] [Google Scholar]




