Abstract
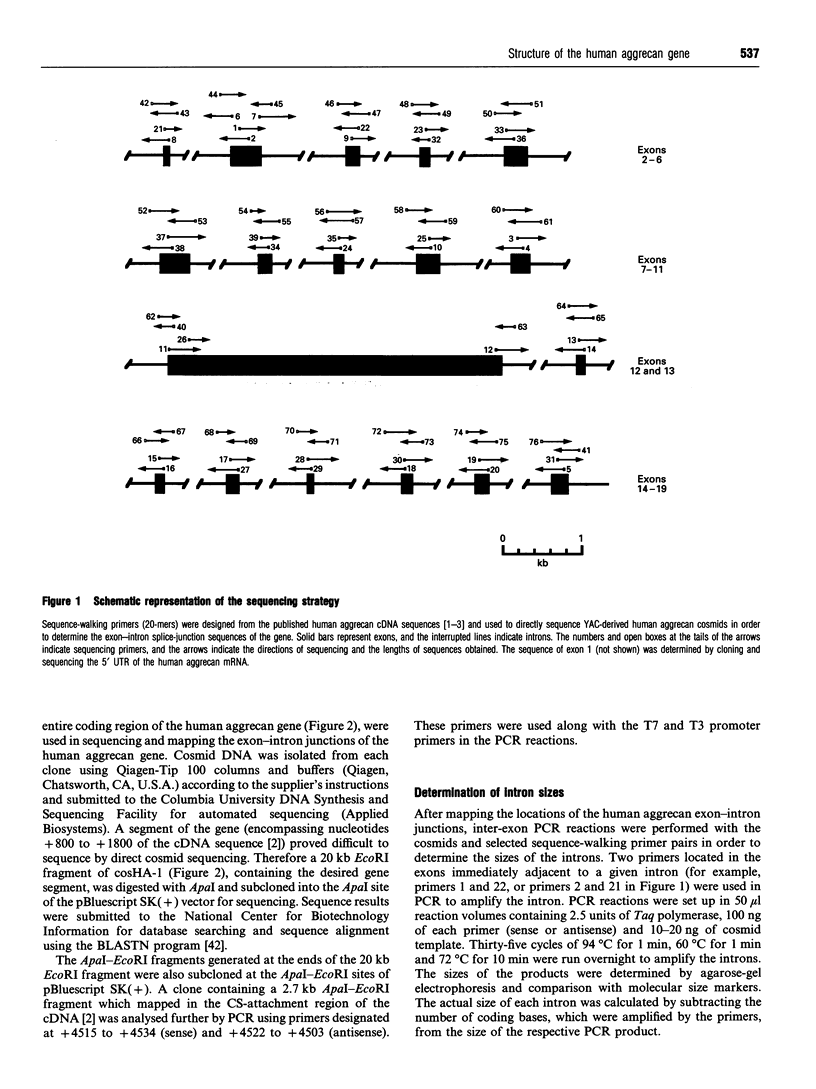
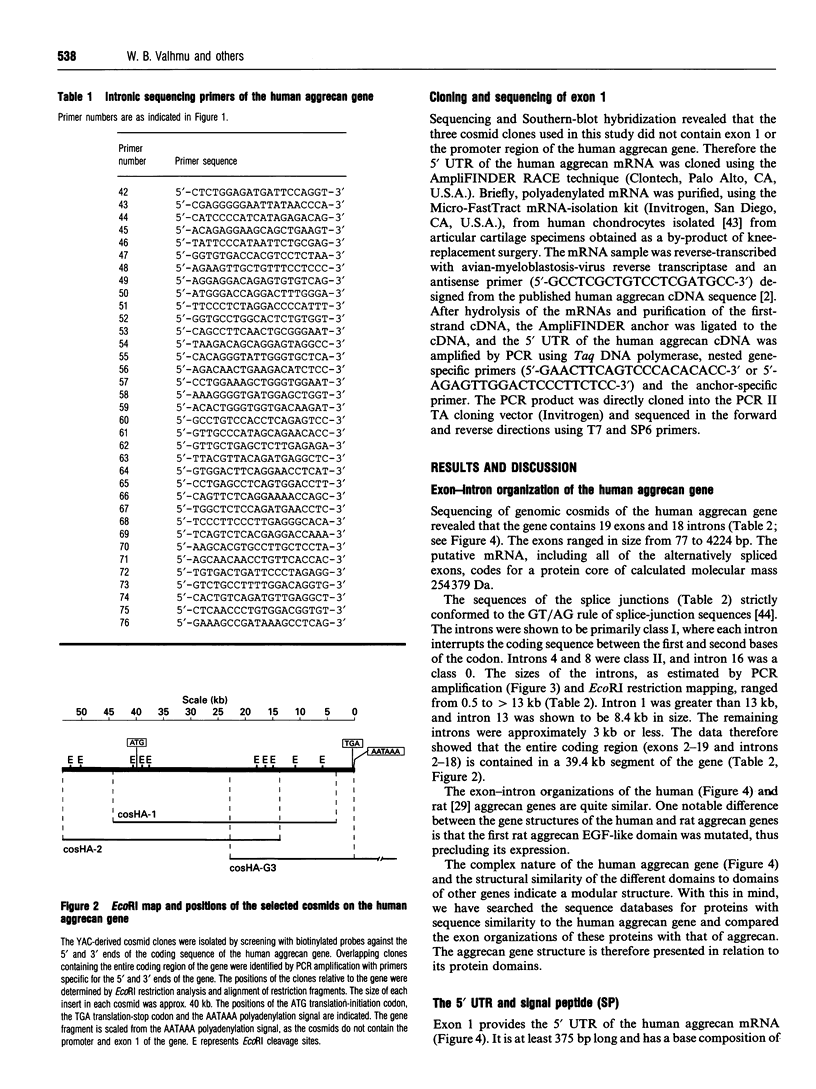
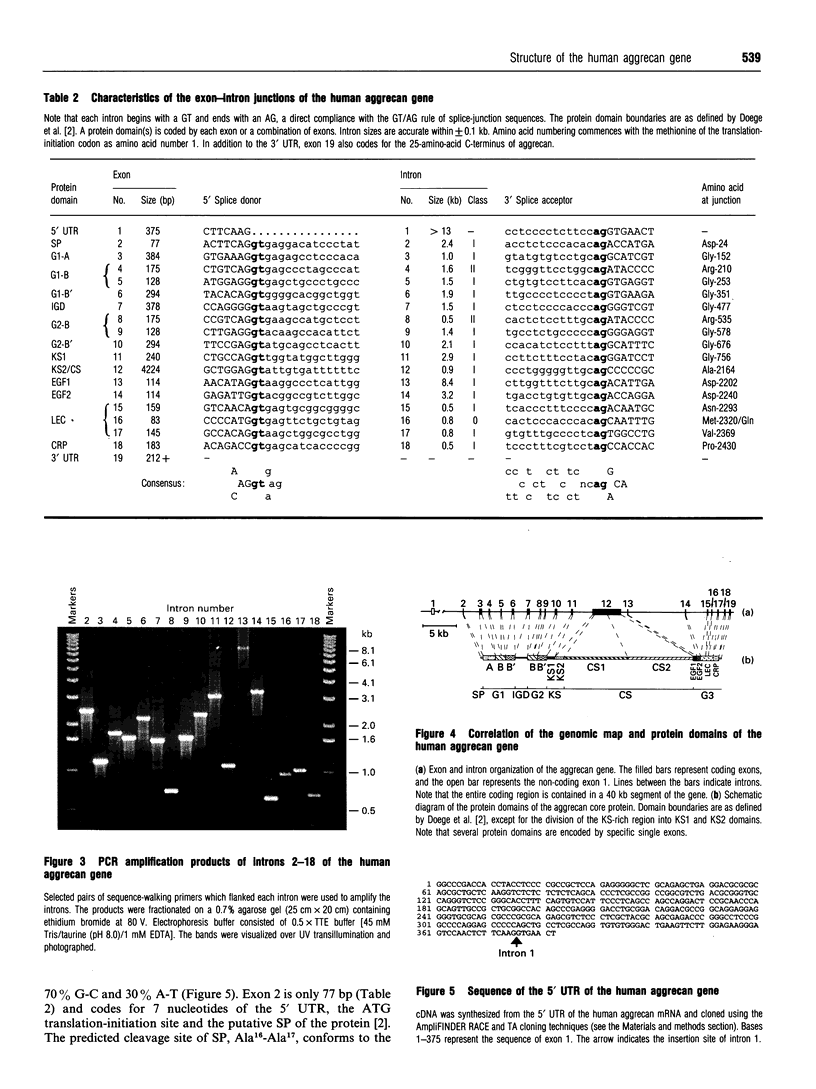
The complete exon-intron organization of the human aggrecan gene has been defined, and the exon organization has been compared with the individual domains of the protein core. A yeast artificial chromosome containing the aggrecan gene was selected from the Centre d'Etude du Polymorphisme Humaine yeast artificial chromosome library. A cosmid sulibrary was created from this, and direct sequencing of individual cosmids was used to provide the exon-intron organization. The human aggrecan gene was found to be composed of 19 exons ranging in size from 77 to 4224 bp. Exon 1 is non-coding, whereas exons 2-19 code for a protein core of 2454 amino acids with a calculated mass of 254379 Da. Intron 1 of the gene is at least 13 kb. Overall, the sizes of the 18 introns range from 0.5 to greater than 13 kb. Each intron begins with a GT and ends with an AG, thus obeying the GT/AG rule of splice-junction sequences. The entire coding region is contained in 39.4 kb of the gene. The organization of exons is strongly related to the specific domains of the protein core. The A loop of G1 and the interglobular domain are encoded by exons 3 and 7 respectively. The B and B' loops of G1 are encoded by exons 4-6, and those of G2 are encoded by exons 8-10. These sets of exons, coding for the B and B' loops, are identical in size and organization. This is supported by the intron classes associated with these exons. Exon 11 codes for the 5' half of the keratan sulphate-rich region, and exon 12 codes for the 3' half of the keratan sulphate-rich region as well as the entire chondroitin sulphate-rich region. G3 is encoded by exons 13-18, including the alternatively spliced epidermal growth factor-like and complement regulatory protein-like domains. The correspondence between the exon organization and the protein domains argues strongly for modular assembly of the aggrecan gene.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht J. C., Nicholas J., Biller D., Cameron K. R., Biesinger B., Newman C., Wittmann S., Craxton M. A., Coleman H., Fleckenstein B. Primary structure of the herpesvirus saimiri genome. J Virol. 1992 Aug;66(8):5047–5058. doi: 10.1128/jvi.66.8.5047-5058.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Antonsson P., Heinegård D., Oldberg A. The keratan sulfate-enriched region of bovine cartilage proteoglycan consists of a consecutively repeated hexapeptide motif. J Biol Chem. 1989 Sep 25;264(27):16170–16173. [PubMed] [Google Scholar]
- Ayté J., Gil-Gómez G., Hegardt F. G. Structural characterization of the 3' noncoding region of the gene encoding rat mitochondrial 3-hydroxy-3-methylglutaryl coenzyme A synthase. Gene. 1993 Jan 30;123(2):267–270. doi: 10.1016/0378-1119(93)90136-q. [DOI] [PubMed] [Google Scholar]
- Barta E., Deák F., Kiss I. Evolution of the hyaluronan-binding module of link protein. Biochem J. 1993 Jun 15;292(Pt 3):947–949. doi: 10.1042/bj2920947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker-André M., Hooft van Huijsduijnen R., Losberger C., Whelan J., Delamarter J. F. Murine endothelial leukocyte-adhesion molecule 1 is a close structural and functional homologue of the human protein. Eur J Biochem. 1992 Jun 1;206(2):401–411. doi: 10.1111/j.1432-1033.1992.tb16940.x. [DOI] [PubMed] [Google Scholar]
- Bezouska K., Crichlow G. V., Rose J. M., Taylor M. E., Drickamer K. Evolutionary conservation of intron position in a subfamily of genes encoding carbohydrate-recognition domains. J Biol Chem. 1991 Jun 25;266(18):11604–11609. [PubMed] [Google Scholar]
- Caterson B., Mahmoodian F., Sorrell J. M., Hardingham T. E., Bayliss M. T., Carney S. L., Ratcliffe A., Muir H. Modulation of native chondroitin sulphate structure in tissue development and in disease. J Cell Sci. 1990 Nov;97(Pt 3):411–417. doi: 10.1242/jcs.97.3.411. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran L., Tanzer M. L. Molecular cloning of chicken aggrecan. Structural analyses. Biochem J. 1992 Dec 15;288(Pt 3):903–910. doi: 10.1042/bj2880903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins T., Williams A., Johnston G. I., Kim J., Eddy R., Shows T., Gimbrone M. A., Jr, Bevilacqua M. P. Structure and chromosomal location of the gene for endothelial-leukocyte adhesion molecule 1. J Biol Chem. 1991 Feb 5;266(4):2466–2473. [PubMed] [Google Scholar]
- Dahlbäck B., Hildebrand B., Linse S. Novel type of very high affinity calcium-binding sites in beta-hydroxyasparagine-containing epidermal growth factor-like domains in vitamin K-dependent protein S. J Biol Chem. 1990 Oct 25;265(30):18481–18489. [PubMed] [Google Scholar]
- Dallo S. F., Chavoya A., Baseman J. B. Characterization of the gene for a 30-kilodalton adhesion-related protein of Mycoplasma pneumoniae. Infect Immun. 1990 Dec;58(12):4163–4165. doi: 10.1128/iai.58.12.4163-4165.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dausset J., Ougen P., Abderrahim H., Billault A., Sambucy J. L., Cohen D., Le Paslier D. The CEPH YAC library. Behring Inst Mitt. 1992 Apr;(91):13–20. [PubMed] [Google Scholar]
- Dennis J. E., Carrino D. A., Schwartz N. B., Caplan A. I. Ultrastructural characterization of embryonic chick cartilage proteoglycan core protein and the mapping of a monoclonal antibody epitope. J Biol Chem. 1990 Jul 15;265(20):12098–12103. [PubMed] [Google Scholar]
- Doege K. J., Garrison K., Coulter S. N., Yamada Y. The structure of the rat aggrecan gene and preliminary characterization of its promoter. J Biol Chem. 1994 Nov 18;269(46):29232–29240. [PubMed] [Google Scholar]
- Doege K. J., Sasaki M., Kimura T., Yamada Y. Complete coding sequence and deduced primary structure of the human cartilage large aggregating proteoglycan, aggrecan. Human-specific repeats, and additional alternatively spliced forms. J Biol Chem. 1991 Jan 15;266(2):894–902. [PubMed] [Google Scholar]
- Doege K., Hassell J. R., Caterson B., Yamada Y. Link protein cDNA sequence reveals a tandemly repeated protein structure. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3761–3765. doi: 10.1073/pnas.83.11.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doege K., Sasaki M., Horigan E., Hassell J. R., Yamada Y. Complete primary structure of the rat cartilage proteoglycan core protein deduced from cDNA clones. J Biol Chem. 1987 Dec 25;262(36):17757–17767. [PubMed] [Google Scholar]
- Doege K., Sasaki M., Yamada Y. Rat and human cartilage proteoglycan (aggrecan) gene structure. Biochem Soc Trans. 1990 Apr;18(2):200–202. doi: 10.1042/bst0180200. [DOI] [PubMed] [Google Scholar]
- Evans G. A., Lewis K., Rothenberg B. E. High efficiency vectors for cosmid microcloning and genomic analysis. Gene. 1989 Jun 30;79(1):9–20. doi: 10.1016/0378-1119(89)90088-7. [DOI] [PubMed] [Google Scholar]
- Ezekowitz R. A., Sastry K., Bailly P., Warner A. Molecular characterization of the human macrophage mannose receptor: demonstration of multiple carbohydrate recognition-like domains and phagocytosis of yeasts in Cos-1 cells. J Exp Med. 1990 Dec 1;172(6):1785–1794. doi: 10.1084/jem.172.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingeroth J. D. Comparative structure and evolution of murine CR2. The homolog of the human C3d/EBV receptor (CD21). J Immunol. 1990 May 1;144(9):3458–3467. [PubMed] [Google Scholar]
- Fosang A. J., Hardingham T. E. Isolation of the N-terminal globular protein domains from cartilage proteoglycans. Identification of G2 domain and its lack of interaction with hyaluronate and link protein. Biochem J. 1989 Aug 1;261(3):801–809. doi: 10.1042/bj2610801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fülöp C., Walcz E., Valyon M., Glant T. T. Expression of alternatively spliced epidermal growth factor-like domains in aggrecans of different species. Evidence for a novel module. J Biol Chem. 1993 Aug 15;268(23):17377–17383. [PubMed] [Google Scholar]
- Gan S. Q., McBride O. W., Idler W. W., Markova N., Steinert P. M. Organization, structure, and polymorphisms of the human profilaggrin gene. Biochemistry. 1990 Oct 9;29(40):9432–9440. doi: 10.1021/bi00492a018. [DOI] [PubMed] [Google Scholar]
- Gerstein R. M., Frankel W. N., Hsieh C. L., Durdik J. M., Rath S., Coffin J. M., Nisonoff A., Selsing E. Isotype switching of an immunoglobulin heavy chain transgene occurs by DNA recombination between different chromosomes. Cell. 1990 Nov 2;63(3):537–548. doi: 10.1016/0092-8674(90)90450-s. [DOI] [PubMed] [Google Scholar]
- Goldstein L. A., Zhou D. F., Picker L. J., Minty C. N., Bargatze R. F., Ding J. F., Butcher E. C. A human lymphocyte homing receptor, the hermes antigen, is related to cartilage proteoglycan core and link proteins. Cell. 1989 Mar 24;56(6):1063–1072. doi: 10.1016/0092-8674(89)90639-9. [DOI] [PubMed] [Google Scholar]
- Gulcher J. R., Nies D. E., Alexakos M. J., Ravikant N. A., Sturgill M. E., Marton L. S., Stefansson K. Structure of the human hexabrachion (tenascin) gene. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9438–9442. doi: 10.1073/pnas.88.21.9438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handford P. A., Mayhew M., Baron M., Winship P. R., Campbell I. D., Brownlee G. G. Key residues involved in calcium-binding motifs in EGF-like domains. Nature. 1991 May 9;351(6322):164–167. doi: 10.1038/351164a0. [DOI] [PubMed] [Google Scholar]
- Hardingham T. E., Fosang A. J. Proteoglycans: many forms and many functions. FASEB J. 1992 Feb 1;6(3):861–870. [PubMed] [Google Scholar]
- Harris N., Super M., Rits M., Chang G., Ezekowitz R. A. Characterization of the murine macrophage mannose receptor: demonstration that the downregulation of receptor expression mediated by interferon-gamma occurs at the level of transcription. Blood. 1992 Nov 1;80(9):2363–2373. [PubMed] [Google Scholar]
- Hayes P. H., Scott G. K., Ng N. F., Hew C. L., Davies P. L. Cystine-rich type II antifreeze protein precursor is initiated from the third AUG codon of its mRNA. J Biol Chem. 1989 Nov 5;264(31):18761–18767. [PubMed] [Google Scholar]
- Heinegård D., Hascall V. C. Aggregation of cartilage proteoglycans. 3. Characteristics of the proteins isolated from trypsin digests of aggregates. J Biol Chem. 1974 Jul 10;249(13):4250–4256. [PubMed] [Google Scholar]
- Heinegård D., Hascall V. C. Characterization of chondroitin sulfate isolated from trypsin-chymotrypsin digests of cartilage proteoglycans. Arch Biochem Biophys. 1974 Nov;165(1):427–441. doi: 10.1016/0003-9861(74)90182-9. [DOI] [PubMed] [Google Scholar]
- Heinegård D., Oldberg A. Structure and biology of cartilage and bone matrix noncollagenous macromolecules. FASEB J. 1989 Jul;3(9):2042–2051. doi: 10.1096/fasebj.3.9.2663581. [DOI] [PubMed] [Google Scholar]
- Hourcade D., Holers V. M., Atkinson J. P. The regulators of complement activation (RCA) gene cluster. Adv Immunol. 1989;45:381–416. doi: 10.1016/s0065-2776(08)60697-5. [DOI] [PubMed] [Google Scholar]
- Hull G. A., Halford N. G., Kreis M., Shewry P. R. Isolation and characterisation of genes encoding rye prolamins containing a highly repetitive sequence motif. Plant Mol Biol. 1991 Nov;17(5):1111–1115. doi: 10.1007/BF00037153. [DOI] [PubMed] [Google Scholar]
- Iozzo R. V., Naso M. F., Cannizzaro L. A., Wasmuth J. J., McPherson J. D. Mapping of the versican proteoglycan gene (CSPG2) to the long arm of human chromosome 5 (5q12-5q14). Genomics. 1992 Dec;14(4):845–851. doi: 10.1016/s0888-7543(05)80103-x. [DOI] [PubMed] [Google Scholar]
- Ishikawa Y., Chin J. E., Hubbard H. L., Wuthier R. E. Utilization and formation of amino acids by chicken epiphyseal chondrocytes: comparative studies with cultured cells and native cartilage tissue. J Cell Physiol. 1985 Apr;123(1):79–88. doi: 10.1002/jcp.1041230113. [DOI] [PubMed] [Google Scholar]
- Jaworski D. M., Kelly G. M., Hockfield S. BEHAB, a new member of the proteoglycan tandem repeat family of hyaluronan-binding proteins that is restricted to the brain. J Cell Biol. 1994 Apr;125(2):495–509. doi: 10.1083/jcb.125.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just W., Klett C., Vetter U., Vogel W. Assignment of the human aggrecan gene AGC1 to 15q25-->q26.2 by in situ hybridization. Hum Genet. 1993 Nov;92(5):516–518. doi: 10.1007/BF00216462. [DOI] [PubMed] [Google Scholar]
- Kiss I., Deák F., Holloway R. G., Jr, Delius H., Mebust K. A., Frimberger E., Argraves W. S., Tsonis P. A., Winterbottom N., Goetinck P. F. Structure of the gene for cartilage matrix protein, a modular protein of the extracellular matrix. Exon/intron organization, unusual splice sites, and relation to alpha chains of beta 2 integrins, von Willebrand factor, complement factors B and C2, and epidermal growth factor. J Biol Chem. 1989 May 15;264(14):8126–8134. [PubMed] [Google Scholar]
- Kiss I., Deák F., Mestrić S., Delius H., Soos J., Dékány K., Argraves W. S., Sparks K. J., Goetinck P. F. Structure of the chicken link protein gene: exons correlate with the protein domains. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6399–6403. doi: 10.1073/pnas.84.18.6399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenberg J. R., Chen X. N., Doege K., Grover J., Roughley P. J. Assignment of the human aggrecan gene (AGC1) to 15q26 using fluorescence in situ hybridization analysis. Genomics. 1993 May;16(2):546–548. doi: 10.1006/geno.1993.1228. [DOI] [PubMed] [Google Scholar]
- Lee T. H., Wisniewski H. G., Vilcek J. A novel secretory tumor necrosis factor-inducible protein (TSG-6) is a member of the family of hyaluronate binding proteins, closely related to the adhesion receptor CD44. J Cell Biol. 1992 Jan;116(2):545–557. doi: 10.1083/jcb.116.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Schwartz N. B., Vertel B. M. cDNA cloning of chick cartilage chondroitin sulfate (aggrecan) core protein and identification of a stop codon in the aggrecan gene associated with the chondrodystrophy, nanomelia. J Biol Chem. 1993 Nov 5;268(31):23504–23511. [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez R., Lynch R. G. CD23 isoforms in murine T and B lymphocytes. Pathobiology. 1993;61(3-4):128–137. doi: 10.1159/000163781. [DOI] [PubMed] [Google Scholar]
- Ord D. C., Ernst T. J., Zhou L. J., Rambaldi A., Spertini O., Griffin J., Tedder T. F. Structure of the gene encoding the human leukocyte adhesion molecule-1 (TQ1, Leu-8) of lymphocytes and neutrophils. J Biol Chem. 1990 May 15;265(14):7760–7767. [PubMed] [Google Scholar]
- Paul M. S., Aegerter M., Cepek K., Miller M. D., Weis J. H. The murine complement receptor gene family. III. The genomic and transcriptional complexity of the Crry and Crry-ps genes. J Immunol. 1990 Mar 1;144(5):1988–1996. [PubMed] [Google Scholar]
- Paulsson M., Yurchenco P. D., Ruben G. C., Engel J., Timpl R. Structure of low density heparan sulfate proteoglycan isolated from a mouse tumor basement membrane. J Mol Biol. 1987 Sep 20;197(2):297–313. doi: 10.1016/0022-2836(87)90125-2. [DOI] [PubMed] [Google Scholar]
- Perkins S. J., Nealis A. S., Dudhia J., Hardingham T. E. Immunoglobulin fold and tandem repeat structures in proteoglycan N-terminal domains and link protein. J Mol Biol. 1989 Apr 20;206(4):737–753. doi: 10.1016/0022-2836(89)90580-9. [DOI] [PubMed] [Google Scholar]
- Perkins S. J., Nealis A. S., Dunham D. G., Hardingham T. E., Muir I. H. Molecular modeling of the multidomain structures of the proteoglycan binding region and the link protein of cartilage by neutron and synchrotron X-ray scattering. Biochemistry. 1991 Nov 5;30(44):10708–10716. doi: 10.1021/bi00108a015. [DOI] [PubMed] [Google Scholar]
- Philippsen P., Stotz A., Scherf C. DNA of Saccharomyces cerevisiae. Methods Enzymol. 1991;194:169–182. doi: 10.1016/0076-6879(91)94014-4. [DOI] [PubMed] [Google Scholar]
- Rauch U., Karthikeyan L., Maurel P., Margolis R. U., Margolis R. K. Cloning and primary structure of neurocan, a developmentally regulated, aggregating chondroitin sulfate proteoglycan of brain. J Biol Chem. 1992 Sep 25;267(27):19536–19547. [PubMed] [Google Scholar]
- Rhodes C., Doege K., Sasaki M., Yamada Y. Alternative splicing generates two different mRNA species for rat link protein. J Biol Chem. 1988 May 5;263(13):6063–6067. [PubMed] [Google Scholar]
- Richards M. L., Katz D. H., Liu F. T. Complete genomic sequence of the murine low affinity Fc receptor for IgE. Demonstration of alternative transcripts and conserved sequence elements. J Immunol. 1991 Aug 1;147(3):1067–1074. [PubMed] [Google Scholar]
- Sandy J. D., Adams M. E., Billingham M. E., Plaas A., Muir H. In vivo and in vitro stimulation of chondrocyte biosynthetic activity in early experimental osteoarthritis. Arthritis Rheum. 1984 Apr;27(4):388–397. doi: 10.1002/art.1780270405. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I., Amiot M., Pesando J. M., Seed B. A lymphocyte molecule implicated in lymph node homing is a member of the cartilage link protein family. Cell. 1989 Mar 24;56(6):1057–1062. doi: 10.1016/0092-8674(89)90638-7. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Har-el R., Tanzer M. L. Partial structure of the gene for chicken cartilage proteoglycan core protein. J Biol Chem. 1988 Oct 25;263(30):15831–15835. [PubMed] [Google Scholar]
- Upholt W. B., Chandrasekaran L., Tanzer M. L. Molecular cloning and analysis of the protein modules of aggrecans. Experientia. 1993 May 15;49(5):384–392. doi: 10.1007/BF01923583. [DOI] [PubMed] [Google Scholar]
- Vertel B. M., Walters L. M., Grier B., Maine N., Goetinck P. F. Nanomelic chondrocytes synthesize, but fail to translocate, a truncated aggrecan precursor. J Cell Sci. 1993 Mar;104(Pt 3):939–948. doi: 10.1242/jcs.104.3.939. [DOI] [PubMed] [Google Scholar]
- Vik D. P., Wong W. W. Structure of the gene for the F allele of complement receptor type 1 and sequence of the coding region unique to the S allele. J Immunol. 1993 Dec 1;151(11):6214–6224. [PubMed] [Google Scholar]
- Watanabe H., Kimata K., Line S., Strong D., Gao L. Y., Kozak C. A., Yamada Y. Mouse cartilage matrix deficiency (cmd) caused by a 7 bp deletion in the aggrecan gene. Nat Genet. 1994 Jun;7(2):154–157. doi: 10.1038/ng0694-154. [DOI] [PubMed] [Google Scholar]
- Wetmore L. A., Gerard C., Drazen J. M. Human lung expresses unique gamma-glutamyl transpeptidase transcripts. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7461–7465. doi: 10.1073/pnas.90.16.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemann H., Paulsson M., Timpl R., Engel J., Heinegård D. Domain structure of cartilage proteoglycans revealed by rotary shadowing of intact and fragmented molecules. Biochem J. 1984 Nov 15;224(1):331–333. doi: 10.1042/bj2240331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H., Watanabe K., Shimonaka M., Yamaguchi Y. Molecular cloning of brevican, a novel brain proteoglycan of the aggrecan/versican family. J Biol Chem. 1994 Apr 1;269(13):10119–10126. [PubMed] [Google Scholar]
- Zimmermann D. R., Ruoslahti E. Multiple domains of the large fibroblast proteoglycan, versican. EMBO J. 1989 Oct;8(10):2975–2981. doi: 10.1002/j.1460-2075.1989.tb08447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]



