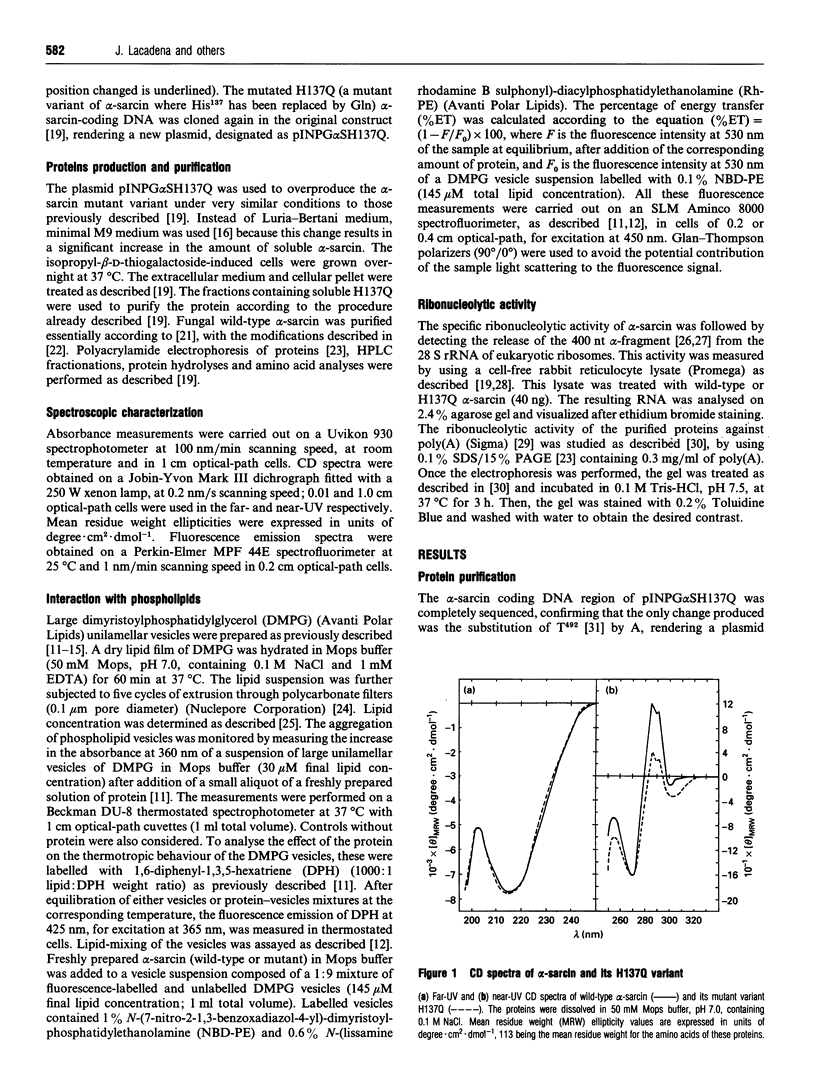
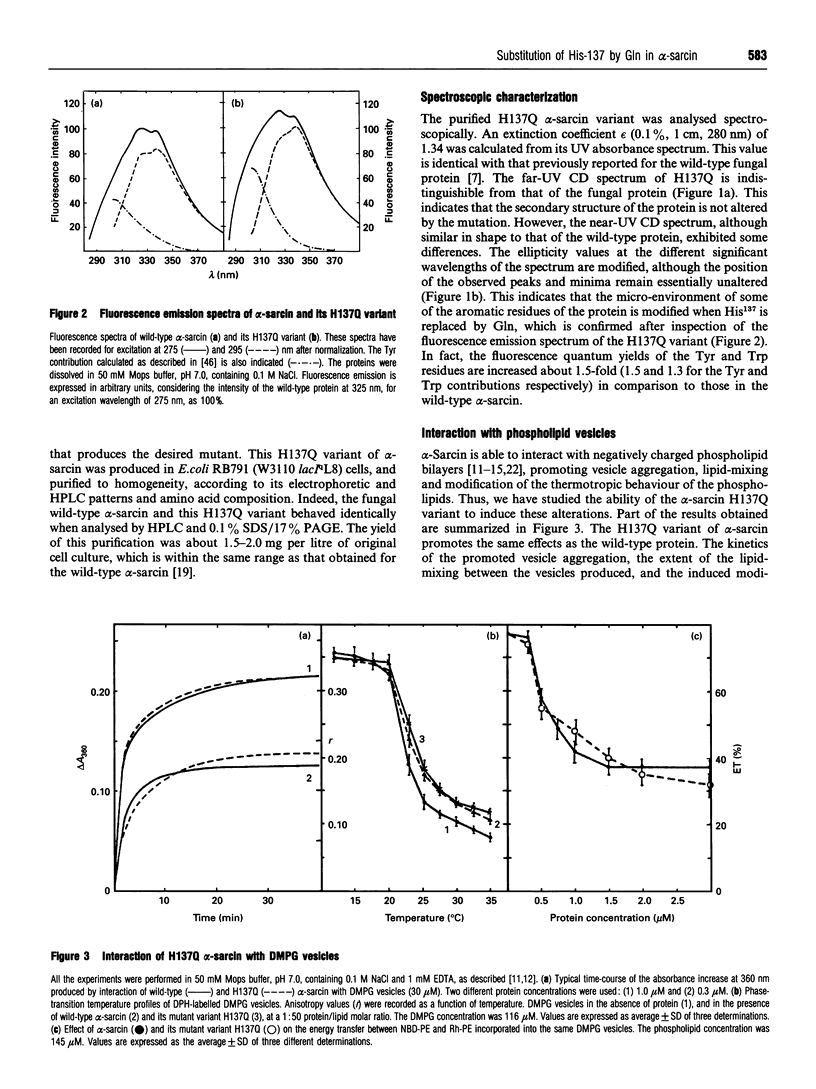
Abstract
The alpha-sarcin cytotoxin is an extracellular fungal protein that inhibits protein biosynthesis by specifically cleaving one phosphodiester bond of the 28 S rRNA. The His137 residue of alpha-sarcin is suggested to be involved in the catalytic activity of this protein, based on the observed sequence similarity with some fungal ribonucleases. Replacement of this residue by Gln (H137Q mutant variant of alpha-sarcin) abolishes the ribonuclease activity of the protein. This has been demonstrated for an homogeneous preparation of the H137Q alpha-sarcin by measuring its effect against both intact rabbit ribosomes and the homopolymer poly(A). The conformation of H137Q alpha-sarcin is highly similar to that of the wild-type protein, which has been analysed by CD and fluorescence spectroscopy. Both H137Q and wild-type alpha-sarcin exhibit identical CD spectra in the peptide-bond region, indicating that no changes at the level of the secondary structure are produced upon mutation. Only minor differences are observed in both near-UV CD and fluorescence emission spectra in comparison to those of the wild-type protein. Moreover, H137Q alpha-sarcin interacts with phospholipid vesicles, promoting the same effects as the native cytotoxin. Therefore, we propose that His137 is part of the ribonucleolytic active site of the cytotoxin alpha-sarcin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Blank A., Sugiyama R. H., Dekker C. A. Activity staining of nucleolytic enzymes after sodium dodecyl sulfate-polyacrylamide gel electrophoresis: use of aqueous isopropanol to remove detergent from gels. Anal Biochem. 1982 Mar 1;120(2):267–275. doi: 10.1016/0003-2697(82)90347-5. [DOI] [PubMed] [Google Scholar]
- Brandhorst T., Yang R., Kenealy W. R. Heterologous expression of the cytotoxin restriction in Aspergillus nidulans and Aspergillus niger. Protein Expr Purif. 1994 Oct;5(5):486–497. doi: 10.1006/prep.1994.1068. [DOI] [PubMed] [Google Scholar]
- Breslow E., Girotti A. W. The interaction of ribonuclease with metal ions. I. Studies of cupric and zinc ions and the effect of cytidylic acid. J Biol Chem. 1966 Dec 10;241(23):5651–5660. [PubMed] [Google Scholar]
- Egami F., Oshima T., Uchida T. Specific interaction of base-specific nucleases with nucleosides and nucleotides. Mol Biol Biochem Biophys. 1980;32:250–277. doi: 10.1007/978-3-642-81503-4_21. [DOI] [PubMed] [Google Scholar]
- Endo Y., Glück A., Chan Y. L., Tsurugi K., Wool I. G. RNA-protein interaction. An analysis with RNA oligonucleotides of the recognition by alpha-sarcin of a ribosomal domain critical for function. J Biol Chem. 1990 Feb 5;265(4):2216–2222. [PubMed] [Google Scholar]
- Endo Y., Huber P. W., Wool I. G. The ribonuclease activity of the cytotoxin alpha-sarcin. The characteristics of the enzymatic activity of alpha-sarcin with ribosomes and ribonucleic acids as substrates. J Biol Chem. 1983 Feb 25;258(4):2662–2667. [PubMed] [Google Scholar]
- Endo Y., Wool I. G. The site of action of alpha-sarcin on eukaryotic ribosomes. The sequence at the alpha-sarcin cleavage site in 28 S ribosomal ribonucleic acid. J Biol Chem. 1982 Aug 10;257(15):9054–9060. [PubMed] [Google Scholar]
- Fernandez-Luna J. L., López-Otin C., Soriano F., Méndez E. Complete amino acid sequence of the Aspergillus cytotoxin mitogillin. Biochemistry. 1985 Feb 12;24(4):861–867. doi: 10.1021/bi00325a008. [DOI] [PubMed] [Google Scholar]
- Gasset M., Martinez del Pozo A., Oñaderra M., Gavilanes J. G. Study of the interaction between the antitumour protein alpha-sarcin and phospholipid vesicles. Biochem J. 1989 Mar 1;258(2):569–575. doi: 10.1042/bj2580569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasset M., Oñaderra M., Goormaghtigh E., Gavilanes J. G. Acid phospholipid vesicles produce conformational changes on the antitumour protein alpha-sarcin. Biochim Biophys Acta. 1991 Oct 11;1080(1):51–58. doi: 10.1016/0167-4838(91)90111-c. [DOI] [PubMed] [Google Scholar]
- Gasset M., Oñaderra M., Martínez del Pozo A., Schiavo G. P., Laynez J., Usobiaga P., Gavilanes J. G. Effect of the antitumour protein alpha-sarcin on the thermotropic behaviour of acid phospholipid vesicles. Biochim Biophys Acta. 1991 Sep 10;1068(1):9–16. doi: 10.1016/0005-2736(91)90055-d. [DOI] [PubMed] [Google Scholar]
- Gasset M., Oñaderra M., Thomas P. G., Gavilanes J. G. Fusion of phospholipid vesicles produced by the anti-tumour protein alpha-sarcin. Biochem J. 1990 Feb 1;265(3):815–822. doi: 10.1042/bj2650815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girotti A. W., Breslow E. The interaction of ribonuclease with metal ions. II. Gel filtration studies of cupric ion-ribonuclease interactions. J Biol Chem. 1968 Jan 10;243(1):216–218. [PubMed] [Google Scholar]
- Heinemann U., Saenger W. Specific protein-nucleic acid recognition in ribonuclease T1-2'-guanylic acid complex: an X-ray study. Nature. 1982 Sep 2;299(5878):27–31. doi: 10.1038/299027a0. [DOI] [PubMed] [Google Scholar]
- Inagaki F., Kawano Y., Shimada I., Takahashi K., Miyazawa T. Nuclear magnetic resonance study on the microenvironments of histidine residues of ribonuclease T1 and carboxymethylated ribonuclease T1. J Biochem. 1981 Apr;89(4):1185–1195. [PubMed] [Google Scholar]
- JENNINGS J. C., OLSON B. H., ROGA V., JUNEK A. J., SCHUURMANS D. M. ALPHA SARCIN, A NEW ANTITUMOR AGENT. II. FERMENTATION AND ANTITUMOR SPECTRUM. Appl Microbiol. 1965 May;13:322–326. doi: 10.1128/am.13.3.322-326.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce B. K., Cohn M. Magnetic resonance studies of the interaction of cupric ion with native and modified forms of ribonuclease. J Biol Chem. 1969 Feb 10;244(3):811–821. [PubMed] [Google Scholar]
- Koellner G., Choe H. W., Heinemann U., Grunert H. P., Zouni A., Hahn U., Saenger W. His92Ala mutation in ribonuclease T1 induces segmental flexibility. An X-ray study. J Mol Biol. 1992 Apr 5;224(3):701–713. doi: 10.1016/0022-2836(92)90554-w. [DOI] [PubMed] [Google Scholar]
- Koellner G., Grunert H. P., Landt O., Saenger W. Crystal structure of the Tyr45Trp mutant of ribonuclease T1 in a complex with 2'-adenylic acid. Eur J Biochem. 1991 Oct 1;201(1):199–202. doi: 10.1111/j.1432-1033.1991.tb16274.x. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lacadena J., Martínez del Pozo A., Barbero J. L., Mancheño J. M., Gasset M., Oñaderra M., López-Otín C., Ortega S., García J., Gavilanes J. G. Overproduction and purification of biologically active native fungal alpha-sarcin in Escherichia coli. Gene. 1994 May 3;142(1):147–151. doi: 10.1016/0378-1119(94)90370-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamy B., Davies J. Isolation and nucleotide sequence of the Aspergillus restrictus gene coding for the ribonucleolytic toxin restrictocin and its expression in Aspergillus nidulans: the leader sequence protects producing strains from suicide. Nucleic Acids Res. 1991 Mar 11;19(5):1001–1006. doi: 10.1093/nar/19.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz A., Cordes F., Heinemann U., Saenger W. Evidence for a substrate-binding subsite in ribonuclease T1. Crystal structure of the complex with two guanosines, and model building of the complex with the substrate guanylyl-3',5'-guanosine. J Biol Chem. 1991 Apr 25;266(12):7661–7667. [PubMed] [Google Scholar]
- López-Otín C., Barber D., Fernández-Luna J. L., Soriano F., Méndez E. The primary structure of the cytotoxin restrictocin. Eur J Biochem. 1984 Sep 17;143(3):621–634. doi: 10.1111/j.1432-1033.1984.tb08415.x. [DOI] [PubMed] [Google Scholar]
- Mancheño J. M., Gasset M., Lacadena J., Martínez del Pozo A., Oñaderra M., Gavilanes J. G. Predictive study of the conformation of the cytotoxic protein alpha-sarcin: a structural model to explain alpha-sarcin-membrane interaction. J Theor Biol. 1995 Feb 7;172(3):259–267. doi: 10.1006/jtbi.1995.0022. [DOI] [PubMed] [Google Scholar]
- Mancheño J. M., Gasset M., Lacadena J., Ramón F., Martínez del Pozo A., Oñaderra M., Gavilanes J. G. Kinetic study of the aggregation and lipid mixing produced by alpha-sarcin on phosphatidylglycerol and phosphatidylserine vesicles: stopped-flow light scattering and fluorescence energy transfer measurements. Biophys J. 1994 Sep;67(3):1117–1125. doi: 10.1016/S0006-3495(94)80578-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez del Pozo A., Gasset M., Oñaderra M., Gavilanes J. G. Conformational study of the antitumor protein alpha-sarcin. Biochim Biophys Acta. 1988 Apr 14;953(3):280–288. doi: 10.1016/0167-4838(88)90036-2. [DOI] [PubMed] [Google Scholar]
- Martínez del Pozo A., Gasset M., Oñaderra M., Gavilanes J. G. Effect of divalent cations on structure-function relationships of the antitumor protein alpha-sarcin. Int J Pept Protein Res. 1989 Nov;34(5):416–422. doi: 10.1111/j.1399-3011.1989.tb00711.x. [DOI] [PubMed] [Google Scholar]
- Merola M., Martínez del Pozo A., Ueno H., Recsei P., Di Donato A., Manning J. M., Tanizawa K., Masu Y., Asano S., Tanaka H. Site-directed mutagenesis of the cysteinyl residues and the active-site serine residue of bacterial D-amino acid transaminase. Biochemistry. 1989 Jan 24;28(2):505–509. doi: 10.1021/bi00428a014. [DOI] [PubMed] [Google Scholar]
- Nonaka T., Nakamura K. T., Uesugi S., Ikehara M., Irie M., Mitsui Y. Crystal structure of ribonuclease Ms (as a ribonuclease T1 homologue) complexed with a guanylyl-3',5'-cytidine analogue. Biochemistry. 1993 Nov 9;32(44):11825–11837. doi: 10.1021/bi00095a011. [DOI] [PubMed] [Google Scholar]
- Oka T., Natori Y., Tanaka S., Tsurugi K., Endo Y. Complete nucleotide sequence of cDNA for the cytotoxin alpha sarcin. Nucleic Acids Res. 1990 Apr 11;18(7):1897–1897. doi: 10.1093/nar/18.7.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oñaderra M., Mancheño J. M., Gasset M., Lacadena J., Schiavo G., Martínez del Pozo A., Gavilanes J. G. Translocation of alpha-sarcin across the lipid bilayer of asolectin vesicles. Biochem J. 1993 Oct 1;295(Pt 1):221–225. doi: 10.1042/bj2950221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco G., Drickamer K., Wool I. G. The primary structure of the cytotoxin alpha-sarcin. J Biol Chem. 1983 May 10;258(9):5811–5818. [PubMed] [Google Scholar]
- Schindler D. G., Davies J. E. Specific cleavage of ribosomal RNA caused by alpha sarcin. Nucleic Acids Res. 1977 Apr;4(4):1097–1110. doi: 10.1093/nar/4.4.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyaert J., Hallenga K., Wyns L., Stanssens P. Histidine-40 of ribonuclease T1 acts as base catalyst when the true catalytic base, glutamic acid-58, is replaced by alanine. Biochemistry. 1990 Sep 25;29(38):9064–9072. doi: 10.1021/bi00490a025. [DOI] [PubMed] [Google Scholar]
- Struck D. K., Hoekstra D., Pagano R. E. Use of resonance energy transfer to monitor membrane fusion. Biochemistry. 1981 Jul 7;20(14):4093–4099. doi: 10.1021/bi00517a023. [DOI] [PubMed] [Google Scholar]
- Szewczak A. A., Moore P. B., Chang Y. L., Wool I. G. The conformation of the sarcin/ricin loop from 28S ribosomal RNA. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9581–9585. doi: 10.1073/pnas.90.20.9581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassylyev D. G., Katayanagi K., Ishikawa K., Tsujimoto-Hirano M., Danno M., Pähler A., Matsumoto O., Matsushima M., Yoshida H., Morikawa K. Crystal structures of ribonuclease F1 of Fusarium moniliforme in its free form and in complex with 2'GMP. J Mol Biol. 1993 Apr 5;230(3):979–996. doi: 10.1006/jmbi.1993.1214. [DOI] [PubMed] [Google Scholar]
- Wool I. G., Glück A., Endo Y. Ribotoxin recognition of ribosomal RNA and a proposal for the mechanism of translocation. Trends Biochem Sci. 1992 Jul;17(7):266–269. doi: 10.1016/0968-0004(92)90407-z. [DOI] [PubMed] [Google Scholar]
- Yang R., Kenealy W. R. Effects of amino-terminal extensions and specific mutations on the activity of restrictocin. J Biol Chem. 1992 Aug 25;267(24):16801–16805. [PubMed] [Google Scholar]