Abstract
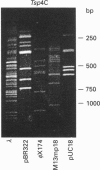
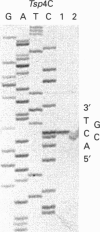
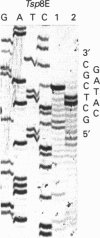
Sixteen isolates of thermophilic bacteria from the genus Thermus, isolated from neutral and alkaline hot water springs in the southwest region of Iceland, were tested for the presence of restriction endonucleases. Extracts from five of the isolates showed evidence of the presence of restriction endonuclease activity by producing discrete nucleotide fragments when incubated at 65 degrees C with lambda phage DNA. Two of the isolates (Tsp4C and Tsp8E) were found to have particularly high levels of restriction endonuclease activity, and the respective enzymes from these two Thermus isolates were partially purified and characterized and their recognition and cleavage sites were determined. Enzyme Tsp4C I is a novel Type II restriction endonuclease recognizing the interrupted palindromic tetranucleotide sequence ACNGT, where N can be any one of the four bases in DNA. Tsp4C I, which retains full enzyme activity when incubated for 10 min at temperatures up to 76 degrees C, hydrolyses the phosphodiester bond in both strands of a double-stranded DNA substrate between the third and fourth bases of the recognition sequence (ACN/GT), generating fragments with a single base 3'-OH overhang. Enzyme Tsp8E I is a thermostable isoschizomer of the mesophilic Type II restriction endonuclease Bgl I (GCCNNNN/NGGC) [Lee, Clanton and Chirikjiam (1979) Fed. Proc. 28, 294], generating fragments with a three base 3'-OH overhang. However, unlike Bgl I, Tsp8E I exhibits considerable thermal stability, retaining full enzyme activity when incubated for 10 min at temperatures up to 78 degrees C. Both Tsp4C I and Tsp8E I represent significant additions to the small but expanding list of the extremely thermostable restriction endonucleases.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barany F. Genetic disease detection and DNA amplification using cloned thermostable ligase. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):189–193. doi: 10.1073/pnas.88.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock T. D., Freeze H. Thermus aquaticus gen. n. and sp. n., a nonsporulating extreme thermophile. J Bacteriol. 1969 Apr;98(1):289–297. doi: 10.1128/jb.98.1.289-297.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N. L., Smith M. A general method for defining restriction enzyme cleavage and recognition sites. Methods Enzymol. 1980;65(1):391–404. doi: 10.1016/s0076-6879(80)65050-2. [DOI] [PubMed] [Google Scholar]
- Degryse E., Glansdorff N., Piérard A. A comparative analysis of extreme thermophilic bacteria belonging to the genus Thermus. Arch Microbiol. 1978 May 30;117(2):189–196. doi: 10.1007/BF00402307. [DOI] [PubMed] [Google Scholar]
- Fuchs C., Rosenvold E. C., Honigman A., Szybalski W. Identification of palindromic sequences recognized by restriction endonucleases, as based on the tabularized sequencing data for seven viral and plasmid DNAs. Gene. 1980 Sep;10(4):357–370. doi: 10.1016/0378-1119(80)90156-0. [DOI] [PubMed] [Google Scholar]
- Lee Y. H., Chirikjian J. G. Sequence-specific endonuclease Bgl I. Modification of lysine and arginine residues of the homogeneous enzyme. J Biol Chem. 1979 Aug 10;254(15):6838–6841. [PubMed] [Google Scholar]
- Meselson M., Yuan R. DNA restriction enzyme from E. coli. Nature. 1968 Mar 23;217(5134):1110–1114. doi: 10.1038/2171110a0. [DOI] [PubMed] [Google Scholar]
- Munster M. J., Munster A. P., Woodrow J. R., Sharp R. J. Isolation and preliminary taxonomic studies of Thermus strains isolated from Yellowstone National Park, USA. J Gen Microbiol. 1986 Jun;132(6):1677–1683. doi: 10.1099/00221287-132-6-1677. [DOI] [PubMed] [Google Scholar]
- Pask-Hughes R., Williams R. A. Extremely thermophilic gram-negative bacteria from hot tap water. J Gen Microbiol. 1975 Jun;88(2):321–328. doi: 10.1099/00221287-88-2-321. [DOI] [PubMed] [Google Scholar]
- Ramaley R. F., Hixson J. Isolation of a nonpigmented, thermophilic bacterium similar to Thermophilic bacterium similar to Thermus aquaticus. J Bacteriol. 1970 Aug;103(2):527–528. doi: 10.1128/jb.103.2.527-528.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. J., Macelis D. REBASE--restriction enzymes and methylases. Nucleic Acids Res. 1993 Jul 1;21(13):3125–3137. doi: 10.1093/nar/21.13.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]