Abstract
Background
Severe cutaneous adverse reactions (SCAR) are a group of delayed presumed T-cell mediated hypersensitivities associated with significant morbidity and mortality. Despite their shared global healthcare burden and impact, the clinical phenotypes, genomic predisposition, drug causality, and treatment outcomes may vary. We describe the establishment and results from the first Australasian registry for SCAR (AUS-SCAR), that via a collaborative network advances strategies for the prevention, diagnosis and treatment of SCAR.
Methods
Prospective multi-center registry of SCAR in Australian adult and adolescents, with planned regional expansion. The registry collects externally verified phenotypic data drug causality, therapeutics and long-term patient outcomes. In addition, biorepository specimens and DNA are collected at participating sites.
Results
we report on the first 100 patients enrolled in the AUS-SCAR database. DRESS (50%) is the most predominant phenotype followed by SJS/TEN (39%) and AGEP (10%), with median age of 52 years old (IQR 37.5, 66) with 1:1 male-to-female ratio. The median latency for all implicated drugs is highly variable but similar for DRESS (median 15 days IQR 5,25) and SJS/TEN (median 21 days, IQR 7,27), while lowest for AGEP (median 2.5 days, IQR 1,8). Antibiotics (54.5%) are more commonly listed as primary implicated drug compare with non-antibiotics agent (45.5%). Mortality rate at 90 days was highest in SJS/TEN at 23.1%, followed by DRESS (4%) and AGEP (0%).
Conclusion
In the first prospective national phenotypic and biorepository of SCAR in the southern hemisphere we demonstrate notable differences to other reported registries; including DRESS-predominant phenotype, varied antibiotic causality and low overall mortality rate. This study also highlights the lack of standardised preventative pharmacogenomic measures and in vitro/in vivo diagnostic strategies to ascertain drug causality.
Trial registration
ANZCTR ACTRN12619000241134. Registered 19 February 2019.
Keywords: Delayed hypersensitivity, T-cell mediated hypersensitivity, Stevens-Johnson syndrome, Toxic epidermal necrolysis, Acute generalized exanthematous pustulosis, Drug reaction with eosinophilia and systemic symptoms, DRESS
Introduction
Severe cutaneous adverse reactions (SCAR), including Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS), acute generalized exanthematous pustulosis (AGEP), and generalized bullous fixed drug eruption (GBFDE) are a group of heterogenous T-cell mediated hypersensitivity reactions1,2 associated with considerable morbidity and mortality.3 At present, there remain several healthcare gaps related to SCAR that might be bridged by multidisciplinary networks.4,5
The pathogenesis of SCAR and other delayed drug hypersensitivities is an aberrant T-cell response, typically to a small molecule medication in a susceptible individual. Patients may be at risk due to an underlying genetic predisposition,6 as one of many important increasingly identified co-factors that result in disease onset. The best recognised drug-HLA association is HLA-B∗57:01 and the abacavir hypersensitivity syndrome that resulted in the implementation of a global pre-prescription screening strategy since 2008.7 Other SCAR-HLA associations include allopurinol DRESS/SJS/TEN (HLA-B∗58:01),8 carbamazepine SJS/TEN (HLA-B∗15:02)9 and vancomycin DRESS (HLA-A∗32:01).10 The understanding of these associations has led to cost-effective screening programs in some settings. However, there is a need to understand the incomplete positive and negative predictive values of HLA testing for medication-associated SCAR that impact clinical translation and knowledge of mechanisms.
Different forms of SCAR differ considerably in their clinical presentation, drug causality and the sensitivity of applied diagnostics.11, 12, 13 A standardized global approach to determine the causative drug by in vivo skin testing after recovery from SCAR and safe prescribing (recommendations as to which drugs to avoid or are safe to use) remains absent. At present, there is an inability to easily ascertain drug causality by current in vivo (eg, skin testing) approaches and this is highly dependent on both the phenotype and culprit drug.14,15 Ex vivo/in vitro immune diagnostics (eg, functional T-cell assays) are not universally available or standardized and remain primarily a research exercise rather than a clinical tool,13,16 hence highlighting a current diagnostic gap in SCAR.14
Healthcare and treatment outcomes have been evaluated in other SCAR registries such as RegiSCAR in Europe17 and the RACGRAD study in Latin America.18 A registry acting as a repository for the collection of epidemiological, clinical, phenotypic and biological samples has not been previously established in Australasia. Further, there are no established SCAR standards of clinical care or national guidelines. Therefore, the establishment of a local clinical registry for SCAR forms a crucial platform to help identify and close the management gaps that currently exist.
In 2019, the Australasian Registry of Severe Cutaneous Adverse Reactions (AUS-SCAR)19 was established to capture SCAR in the inpatient Australian setting with planned regional expansion, to better define the morbidity and mortality from SCAR, and inform translational research to improve the prevention, diagnosis and management of patients with SCAR. The primary aim of AUS-SCAR is to ensure continued surveillance of new and emerging causative drugs and improvement pharmacovigilance. Further, secondary objectives include defining the epidemiology, host and drug factors that predict treatment outcomes, pharmacogenomic associations and long-term outcomes, including quality of life (QOL) impact. In this initial report we report on the clinical characteristics, including implicated demographics, implicated drug and clinical phenotype of the first 100 patients recruited to the first 14 sites in AUS-SCAR.
Methods
Sites and study participants
AUS-SCAR (ACTRN No. 12619000241134) was established and is hosted by the Centre for Antibiotic Allergy and Research, Austin Health, Melbourne, Australia. The project was reviewed and approved by the Austin Health Human Research Ethics Committee (Reference: HREC/50791/Austin-2019). The protocol for AUS-SCAR was published in 2022.19 Fourteen tertiary referral hospitals from states or territories around Australia have contributed to the registry to date (Supplementary Table 1). In brief, all participating sites are adult tertiary referral centres, 4 of which included designated regional burns referral centres. The burns centres in these regions manage all cases of SJS, TEN, and SJS/TEN overlap due to the extensive skin loss and surgical management required.
Registry structure, data collection and biospecimens
The AUS-SCAR registry components, data collection, biospecimen recovery model and governance was previously published.19 In brief, AUS-SCAR is overseen by a steering committee chaired by an investigator not associated with a clinical site, with broad multidisciplinary representation. At a patient level, following informed written consent from the participant or medical decision maker (MDM) (if applicable), prospective demographic, phenotypic, causality, treatment and outcome data were collected, as was saliva for DNA isolation.19 A waiver of consent was approved for retrospective case inclusion for patients who died prior to obtaining consent. A 12-month quality of life (QOL) survey using a validated drug allergy questionnaire was also undertaken. At selected participating sites with sample collection capacity, additional biological samples were collected including peripheral blood mononuclear cells (PBMCs), skin tissue, blister fluid, and paraffin embedded sections. Blister fluid and PBMCs were utilised for ex vivo diagnostics as per previously published methods.19, 20, 21
Case definitions and validation
Cases were considered eligible for inclusion if the phenotype was consistent with published definitions,19 agreed upon by 2 participating site investigators, and adjudicated by a dermatologist or immunologist, or proven on biopsy.19 Skin biopsy definitions utilised are provided in Supplementary Methods. Following recruitment, case summaries were reviewed by the Centre for Antibiotic Allergy and Research Staff and presented to 2 independent investigators for external validation and final inclusion into the registry (See Supplementary Figure 1 for case summary example). If available skin testing concentrations deployed are detailed in Supplementary Methods.
Analysis and statistical methods
Statistical analysis was performed using Stata version 17.0 (StataCorp LLC, College Station, TX, USA). Continuous variables were summarized using median and interquartile range (IQR), and categorical variables were summarized using frequency and percentage. Groups were compared using Kruskal Wallis test (continuous variables) or Fisher's Exact test (categorical variables).
Results
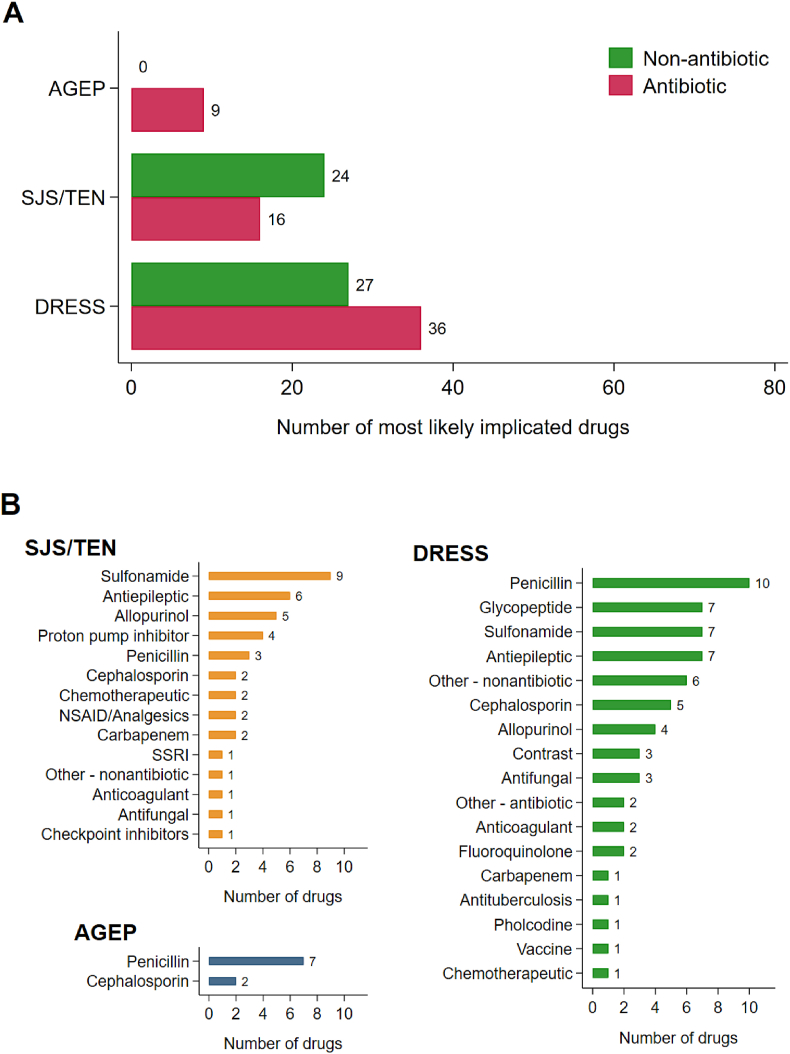
The first 100 patients were recruited from July 2019 to October 2022 across 12 of the 14 participating sites in Victoria (n = 7), New South Wales (n = 3), South Australia (n = 1), and the Northern Territory (n = 1). Patient characteristics are presented overall and stratified for phenotype in Table 1. Forty-five percent of patients had at least 1 biospecimen collected (blood, skin, or blister fluid) and 95% had DNA collected (Supplementary Table 2). The primary implicated drugs stratified for antibiotic and non-antibiotic, and specific class are demonstrated in Fig. 1. All implicated drugs are provided in Supplementary Figure 2. The median latency (time from drug commencement to rash onset) for all implicated drugs are highly variable but are similar for DRESS (median 15 days IQR 5,25) and SJS/TEN (median 21 days, IQR 7,27), while AGEP has lower latency (median 2.5 days, IQR 1,8). Further, individual site investigator causality assessments and individual implicated drugs are shown in Supplementary Tables 3 and 4, respectively. Patients with DRESS comprised 50% of the cohort, 39% with SJS/TEN, and 10% with AGEP. The median age was 52 years (interquartile range, IQR, 37.5, 66.0) with 1:1 male-to-female ratio in the cohort. Patients with SJS/TEN were older with a median age of 58 years, compared to a median 49 years in DRESS and 47 years in AGEP. SJS/TEN patients were more likely to be female (59%), compared with AGEP (40%) and DRESS (44%). The median Charlson comorbidity index was 2 (IQR, 0,4.5.0) and 38% of the cohort was immunocompromised. A summary of Patient Comorbidities Charlson Comorbidity Index and Patient Comorbidities Immunocompromised Status are shown in Supplementary Table 5. Nineteen patients reported a previous skin disorder (Supplementary Table 6). Twenty-one percent of all patients had a label of one or more known prior medication allergies, 4% with a history of the same reaction to same drug (Supplementary Table 4). The median length of stay during the index admission at the participating site was 11 days (IQR, 6,29); this was highest in SJS/TEN patients at 14 days (IQR, 7,27). The median time from rash onset to hospital admission was 5.5 days (IQR, 3,13). Twenty-eight percent of all patients were admitted to an intensive care unit (ICU); this rate was highest in SJS/TEN patients (41%) (Supplementary Table 7).
Table 1.
Patient characteristics, by phenotype
| Overall (n = 100)a | DRESS (n = 50) | SJS/TEN (n = 39) | AGEP (n = 10) | |
|---|---|---|---|---|
| Participating site region/state | ||||
| Victoria | 82 (82%) | 40 (80%) | 34 (87%) | 7 (70%) |
| New South Wales | 9 (9%) | 4 (8%) | 2 (5%) | 3 (30%) |
| South Australia | 6 (6%) | 3 (6%) | 3 (8%) | 0 (0%) |
| Northern Territory | 3 (3%) | 3 (6%) | 0 (0%) | 0 (0%) |
| Sex (n, % female) | 50 (50%) | 22 (44%) | 23 (59%) | 4 (40%) |
| Age (years), median (IQR) | 52 (37.5, 66) | 49 (36, 63) | 58 (43, 69) | 47 (33, 62) |
| Ethnicity | ||||
| Oceanian (including Australian and New Zealand) | 5 (5%) | 2 (4%) | 3 (8%) | 0 (0%) |
| Australian Aboriginal | 3 (3%) | 2 (4%) | 1 (3%) | 0 (0%) |
| Melanesian and Papuan | 1 (1%) | 0 (0%) | 1 (3%) | 0 (0%) |
| Polynesian | 1 (1%) | 0 (0%) | 1 (3%) | 0 (0%) |
| North-West European | 34 (34%) | 16 (32%) | 15 (38%) | 3 (30%) |
| Southern and Eastern European | 10 (10%) | 7 (14%) | 2 (5%) | 1 (10%) |
| European unknown (Caucasian) | 18 (18%) | 9 (18%) | 5 (13%) | 4 (40%) |
| North African and Middle Eastern | 2 (2%) | 1 (2%) | 1 (3%) | 0 (0%) |
| South-east Asian | 11 (11%) | 4 (8%) | 5 (13%) | 2 (20%) |
| North-east Asian | 5 (5%) | 2 (4%) | 2 (5%) | 0 (0%) |
| Southern and Central Asian | 12 (12%) | 7 (14%) | 5 (13%) | 0 (0%) |
| American (north, central, south) | 3 (3%) | 2 (4%) | 1 (3%) | 0 (0%) |
| Sub-Saharan Africa | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Admitting Ward | ||||
| General medical | 52 (52%) | 32 (64%) | 13 (33%) | 6 (60%) |
| General surgical | 7 (7%) | 5 (10%) | 1 (3%) | 1 (10%) |
| Emergency | 3 (3%) | 1 (2%) | 1 (3%) | 1 (10%) |
| Intensive Care Unit | 13 (13%) | 3 (6%) | 9 (23%) | 1 (10%) |
| Burn unit | 10 (10%) | 0 (0%) | 10 (26%) | 0 (0%) |
| Otherb | 15 (15%) | 9 (18%) | 5 (13%) | 1 (10%) |
| ICU admit during admission for SCAR | 28 (28%) | 10 (20%) | 17 (44%) | 1 (10%) |
| Burn unit required during admission | 18 (18%) | 1 (2%) | 17 (44%) | 0 (0%) |
| Any prior immune-mediated drug allergy | 21 (21%) | 9 (18%) | 9 (23%) | 2 (20%) |
| Prior immune-mediated non-antibiotic drug allergy | 11 (11%) | 6 (12%) | 4 (10%) | 0 (0%) |
| Type of prior non-antibiotic allergy | ||||
| Aspirin/NSAID/paracetamol | 3 (3%) | 0 (0%) | 2 (5%) | 0 (0%) |
| Antiepileptic | 1 (1%) | 1 (2%) | 0 (0%) | 0 (0%) |
| Otherc | 7 (7%) | 5 (10%) | 2 (5%) | 0 (0%) |
| Prior immune-mediated antibiotic allergy | 19 (19%) | 8 (16%) | 8 (21%) | 2 (20%) |
| Number of different immune-mediated antibiotic allergies | ||||
| Single | 14 (14%) | 6 (12%) | 6 (15%) | 1 (10%) |
| Multiple | 4 (4%) | 1 (2%) | 2 (5%) | 1 (10%) |
| N/A | 82 (82%) | 43 (86%) | 31 (79%) | 8 (80%) |
| Type of prior immune-mediated antibiotic allergy | ||||
| Penicillin allergy | 13 (13%) | 6 (12%) | 6 (15%) | 1 (10%) |
| Beta-lactam (non-penicillin) allergy | 7 (7%) | 3 (6%) | 2 (5%) | 1 (10%) |
| Sulfonamide allergy | 3 (3%) | 2 (4%) | 1 (3%) | 0 (0%) |
| Age adjusted CCI, median (IQR) | 2 (0, 4.5) | 2 (0, 4) | 3 (0, 6) | 1.5 (0, 3) |
| Immunocompromisedd | 38 (38%) | 18 (36%) | 17 (44%) | 3 (30%) |
| Time in days from index rash to admission at participating site (median, IQR) | 5.5 (3, 13) (n = 98) | 8.5 (4, 17.5) (n = 48) | 5 (3, 11) | 3.5 (2, 5) |
| Resolution of rash <15 days | 25 (25%) | 11 (22%) | 8 (21%) | 6 (60%) |
Abbreviations: AGEP, acute generalized exanthematous pustulosis; SJS, Stevens Johnson Syndrome; TEN, toxic epidermal necrolysis; DRESS, drug reaction with eosinophilia and systemic symptoms; ATSI, Aboriginal and Torres Strait Islanders; IQR, interquartile range; CCI, age-adjusted Charlson Comorbidity Index; NSAID, non-steroidal anti-inflammatory drug.
A single patient of GBFDE (generalized bullous fixed drug eruption) was included in the total cohort but data for the single case are not represented.
Antibiotic Allergy (n = 1), Critical Care Unit (CCU) (n = 1), Cardiology (n = 1), Hospital in the Home (HITH) (n = 1), Haematology (n = 4), Oncology (n = 3), Neurology (n = 1), Orthopaedic (n = 1).
Contrast (n = 1), contrast/tramadol (n = 1), contrast/arsenic (n = 1), olanzapine/quetiapine/hydroxychloroquine (n = 1), Jumper Jack ant (n = 1), lenalidomide (n = 1), perindopril/atorvastatin/rosuvastatin (n = 1).
Immunocompromised – Cancer or haematological malignancy, transplant recipient, autoimmune or inflammatory condition, HIV, splenectomy.
Fig. 1.
Most likely implicated drug classes by phenotype A) Phenotypes stratified for primary implicated antibiotic vs non-antibiotic B) Phenotypes stratified for primary implicated drug class Abbreviations: PPI, proton-pump inhibitors; SSRI, selective serotine receptor inhibitors; NSAID, non-steroidal anti-inflammatory drug Other antibiotic: daptomycin, minocycline Other nonantibiotic: anastrozole, amiodarone, bortezomib Vaccines (n = 8): Comirnaty Pfizer (1), Hep B (1), Meningococcal Group B multicomponent (1), Meningococcal polysaccharide conjugate (1), pneumococcal (1)
Regarding key clinical characteristics, documented fever was most prevalent in DRESS cases (68%), compared with SJS/TEN (33.3%) and AGEP (4%). Mucosal involvement was a predominant sign in SJS/TEN (94.9%) but less frequently reported in DRESS (22%). Facial oedema was noted in 62% of DRESS and 43.6% of SJS/TEN patients. Eosinophilia was present in 86% of DRESS cases, it was also reported in 45.5% of AGEP patients. Rash resolution did not occur until at least 15 days post rash onset for most patients (70%). Internal organ involvement was noted in 54% of SJS/TEN, 86% of DRESS and 30% of AGEP cases. The median RegiSCAR phenotypic score for DRESS was 6 (probable) (IQR 5,6) and validated score for AGEP was 7 (IQR 6,8) (Table 3).
Table 3.
Clinical characteristics tables
| Overall (n = 100) |
DRESS (n = 50) |
SJS/TEN (n = 39) |
AGEP (n = 10) |
|
|---|---|---|---|---|
| Time in days from index rash to admission at participating site, median (IQR) | 5.5 (3, 13) (n = 98) | 8.5 (4, 17.5) (n = 48) | 5 (3, 11) | 3.5 (2, 5) |
| Rash components | ||||
| Erythroderma (≥90% BSA involvement | 20 (20%) | 12 (24%) | 8 (21%) | 0 (0%) |
| Maculopapular | 68 (68%) | 46 (92%) | 17 (44%) | 5 (50%) |
| Urticarial (raised oedematous non-scaly plaques) | 32 (32%) | 19 (38%) | 8 (21%) | 5 (50%) |
| Pustules | 15 (15%) | 5 (10%) | 0 (0%) | 10 (100%) |
| Vesicles, bullae or blisters | 32 (32%) | 6 (12%) | 25 (64%) | 0 (0%) |
| Erosion/crusting | 43 (43%) | 6 (12%) | 37 (95%) | 0 (0%) |
| Typical target lesions (3 zones) | 3 (3%) | 3 (6%) | 0 (0%) | 0 (0%) |
| Atypical targets (2 zones) | 24 (24%) | 5 (10%) | 18 (46%) | 1 (10%) |
| Exfoliative dermatitis/desquamation (superficial epidermal peeling) | 37 (37%) | 19 (38%) | 13 (33%) | 5 (50%) |
| Epidermal detachment | 23 (23%) | 0 (0%) | 22 (56%) | 0 (0%) |
| Distribution of Rash | ||||
| Distal extremities (arms/legs) greater than truck/torso, no mucosal surfaces affected | 5 (5%) | 2 (4%) | 1 (3%) | 2 (20%) |
| Distal extremities (arms/legs) greater than truck/torso, 1 mucosal surface affected (conjunctiva, lips/oral, genitalia) | 3 (3%) | 2 (4%) | 1 (3%) | 0 (0%) |
| Distal extremities (arms/legs) greater than trunk/torso, 2 mucosal surfaces affected | 1 (1%) | 0 (0%) | 1 (3%) | 0 (0%) |
| Distal extremities (arms/legs) greater than trunk/torso, 3 mucosal surfaces affected | 1 (1%) | 0 (0%) | 1 (3%) | 0 (0%) |
| Trunk/torso greater than extremities (arms/legs), no mucosal surfaces affected | 13 (13%) | 9 (18%) | 0 (0%) | 3 (30%) |
| Trunk/torso greater than extremities (arms/legs), 1 mucosal surfaces affected | 7 (7%) | 2 (4%) | 5 (13%) | 0 (0%) |
| Trunk/torso greater than extremities (arms/legs), 2 mucosal surfaces affected | 7 (7%) | 0 (0%) | 7 (18%) | 0 (0%) |
| Trunk/torso greater than extremities (arms/legs), 3 mucosal surfaces affected | 4 (4%) | 0 (0%) | 4 (10%) | 0 (0%) |
| Widespread (>50%), 0 mucosal surfaces affected | 33 (33%) | 28 (56%) | 1 (3%) | 4 (40%) |
| Widespread (>50%), 1 mucosal surface | 11 (11%) | 4 (8%) | 6 (15%) | 1 (10%) |
| Widespread (>50%), 2 mucosal surfaces | 8 (8%) | 2 (4%) | 6 (15%) | 0 (0%) |
| Widespread (>50%), 3 mucosal surfaces affected | 6 (6%) | 0 (0%) | 6 (15%) | 0 (0%) |
| Facial involvement | ||||
| No | 15 (15%) | 7 (14%) | 3 (8%) | 4 (40%) |
| Yes | 83 (83%) | 41 (82%) | 36 (92%) | 6 (60%) |
| Unknown | 2 (2%) | 2 (4%) | 0 (0%) | 0 (0%) |
| Facial oedema | 49 (49%) | 30 (60%) | 16 (41%) | 3 (30%) |
| Scalp involvement | ||||
| No | 47 (47%) | 23 (46%) | 17 (44%) | 6 (60%) |
| Yes | 31 (31%) | 19 (38%) | 11 (28%) | 1 (10%) |
| Unknown | 22 (22%) | 8 (16%) | 11 (28%) | 3 (30%) |
| Palms or sole involvement | ||||
| No | 42 (42%) | 22 (44%) | 12 (31%) | 7 (70%) |
| Yes | 45 (45%) | 21 (42%) | 22 (56%) | 2 (20%) |
| Unknown | 13 (13%) | 7 (14%) | 5 (13%) | 1 (10%) |
| Pruritus | ||||
| No | 17 (17%) | 3 (6%) | 12 (31%) | 1 (10%) |
| Yes | 70 (70%) | 44 (88%) | 19 (49%) | 7 (70%) |
| Unknown | 13 (13%) | 3 (6%) | 8 (21%) | 2 (20%) |
| Fever >38.5 °C on admission [within 48 h of rash onset] | 50 (50%) | 34 (68%) | 12 (31%) | 4 (40%) |
| Internal organ involvement (liver, renal, pulmonary) during admission | 67 (67%) | 43 (86%) | 21 (54%) | 3 (30%) |
| Renal Failure | 21 (21%) | 10 (20%) | 11 (28%) | 0 (0%) |
| Liver failure | 36 (36%) | 27 (54%) | 8 (21%) | 1 (10%) |
| Respiratory failure | 12 (12%) | 4 (8%) | 8 (21%) | 0 (0%) |
| Blood abnormality | ||||
| Platelets <150 × 10ˆ9/L | 24 (24%) | 9 (18%) | 14 (36%) | 1 (10%) |
| WBC <4 × 10ˆ9/L | 15 (15%) | 6 (12%) | 9 (23%) | 0 (0%) |
| Hb < 100 g/L | 40 (40%) | 19 (38%) | 20 (51%) | 1 (10%) |
| Atypical lymphocytes (on blood film) | 15 (15%) | 12 (24%) | 3 (8%) | 0 (0%) |
| Eosinophilia (>0.7 × 10ˆ9/L) | 45 (45%) | 37 (74%) | 4 (10%) | 4 (40%) |
| Resolution <15 days | 25 (25%) | 11 (22%) | 8 (21%) | 6 (60%) |
| History of herpes infection (HSV 1/2) in 1 month prior to onset | ||||
| No | 78 (78%) | 41 (82%) | 28 (72%) | 8 (80%) |
| Yes | 5 (5%) | 1 (2%) | 4 (10%) | 0 (0%) |
| Unknown | 17 (17%) | 8 (16%) | 7 (18%) | 2 (20%) |
| History of ANY infection in 1 month prior to onset | ||||
| No | 39 (39%) | 22 (44%) | 15 (38%) | 2 (20%) |
| Yes | 51 (51%) | 27 (54%) | 16 (41%) | 7 (70%) |
| Unknown | 10 (10%) | 1 (2%) | 8 (20%) | 1 (10%) |
| Known concurrent viraemia (HHV6, CMV, EBV - confirmed by positive blood PCR) | ||||
| No | 60 (60%) | 35 (70%) | 21 (54%) | 3 (30%) |
| Yes | 1 (1%) | 1 (2%) | 0 (0%) | 0 (0%) |
| Unknown | 39 (39%) | 14 (28%) | 18 (49%) | 7 (70%) |
| History of prior skin disorder? | 19 (19%) | 11 (22%) | 6 (15%) | 1 (10%) |
| Phenotype specific: | N (%) |
|---|---|
| DRESS (N = 50) | |
| Fever >38.5 °C | 40 (80%) |
| Enlarged lymph nodes at 2 or more sites > 1 cm | 12 (24%) |
| Atypical lymphocytes | 20 (40%) |
| Eosinophilia (×10ˆ9/L) | |
| No | 7 (14%) |
| 0.7–1.49 | 12 (24%) |
| ≥1.5 | 31 (62%) |
| Rash | |
| ≥ 50% body | 47 (94%) |
| Rash at least 2 of oedema, infiltration, purpura, scalling | 34 (68%) |
| Biopsy suggestive of DRESS | 39 (78%) |
| Pustules | 6 (12%) |
| Blisters | 6 (12%) |
| Number of internal organs involved | |
| 0 | 4 (8%) |
| 1 | 32 (64%) |
| 2 | 13 (26%) |
| 3 | 1 (2%) |
| Internal organ involved | |
| Renal | 19 (38%) |
| Liver | 39 (78%) |
| Pulmonary | 1 (2%) |
| CNS | 1 (2%) |
| Cardiac | 3 (6%) |
| GI track | 1 (2%) |
| Resolution of rash< 15 days | 9 (18%) |
| Biological investigations - More than 3 performed to exclude alternative diagnosis | 47 (94%) |
| RegiSCAR Score, median (IQR) | 6 (5, 6) (n = 49) |
| SJS/TEN (N = 39) | |
| Total estimated BSA of rash | |
| < 10% BSA | 6 (15%) |
| 10–30% BSA | 10 (26%) |
| 30–50% BSA | 2 (5%) |
| 50–90% BSA | 11 (28%) |
| 90–100% BSA (erythroderma) | 8 (21%) |
| Maximum estimated BSA of epidermal detachment (denuded/sloughed) during the course of illness | |
| BSA <10% | 18 (46%) |
| BSA 10–30% | 8 (21%) |
| BSA >30% | 10 (26%) |
| Unknown | 3 (8%) |
| Blistering pattern in SJS/TEN (select best match) (choice = erythema with sloughing | |
| Unchecked | 14 (36%) |
| Checked | 25 (64%) |
| Blisters on dusky macules | 15 (38%) |
| Vesicles (<5 mm)) | 5 (13%) |
| Nikolsky's sign | 21 (54%) |
| Blistering or bullous rash | 31 (79%) |
| Did the patient present with a fever >38 °C | 17 (44%) |
| Heart rate on admission >120 bpm | 10 (26%) |
| Urea on admission >10 mmol/L | 13 (33%) |
| SCORTEN score | |
| 0-1 | 16 (41%) |
| 2 | 6 (15%) |
| 3 | 7 (18%) |
| 4 | 8 (21%) |
| 5+ | 1 (3%) |
| Ophthalmology review | 33 (85%) |
| Gynaecology review | 12 (31%) |
| Urology review | 8 (21%) |
| Has Linear IgA disease, pemphigoid, SSSS, TEN-like lupus, other autoimmune bullous disease been excluded on biopsy | 35 (90%) |
| AGEP (N = 10) | |
| Type of pustules | |
| Follicular | 1 (10%) |
| Non-follicular | 7 (70%) |
| Both | 1 (10%) |
| Unknown | 1 (10%) |
| AGEP extent of erythema/pustules | |
| 10–30% BSA | 2 (20%) |
| 30–50% BSA | 4 (40%) |
| 50–90% BSA | 4 (40%) |
| Distribution | |
| Folds predominate | 3 (30%) |
| Torso | 8 (80%) |
| Limbs | 6 (60%) |
| Widespread | 2 (20%) |
| Head and neck | 2 (20%) |
| AGEP mucosal involvement | 1 (10%) |
| AGEP score, median (IQR) | 7 (6, 8) (n = 6) |
Abbreviations: AGEP, acute generalized oedematous pustulosis; SJS, Stevens Johnson Syndrome; TEN, toxic epidermal necrolysis; DRESS, drug reaction with eosinophilia and systemic symptoms; IQR, interquartile range; BSA, Body Surface Area.
Inpatient mortality was reported in 7% (7/100) of cases, and 57% (4/7) of these cases were deemed attributable to SCAR. Regarding 90-day outcomes, 11% (11/100) of patients died and a further 8% (8/100) reported ongoing disability at 90 days (Table 2). A total of 16 patients experienced a readmission within the 90-day period (Supplementary Table 8). The all-cause mortality rate at 90 days was highest in SJS/TEN at 23.1%, compared with 4% in DRESS and 0% in AGEP. Most patients received specific immunomodulatory treatment with 77% of the cohort receiving systemic corticosteroids and 79% receiving topical corticosteroids. A total of 16 patients were readmitted within 90 days: 20% of DRESS, 10% of SJS/TEN, and 20% of AGEP cases. Whilst a significant number reported specialist follow-up (67%), a smaller proportion reported diagnostic in vivo skin testing following recovery (28%).
Table 2.
Outcome data stratified for phenotype
| Overall (n = 100) | DRESS (n = 50) | SJS/TEN (n = 39) | AGEP (n = 10) | p-value | |
|---|---|---|---|---|---|
| Did the patient receive medical treatment (Corticosteroids [systemic/topical], antiviral) | 95 (95%) | 45 (90%) | 39 (100%) | 10 (100%) | 0.097 |
| Treatment or supportive care | |||||
| Systemic corticosteroids | 77 (77%) | 42 (84%) | 29 (74%) | 5 (50%) | 0.066 |
| Topical corticosteroids | 79 (79%) | 39 (78%) | 30 (77%) | 9 (90%) | 0.82 |
| Antivirals | 8 (8%) | 2 (4%) | 6 (15%) | 0 (0%) | 0.14 |
| Antibiotics | 18 (18%) | 8 (16%) | 9 (23%) | 1 (10%) | 0.64 |
| Paracetamol | 37 (37%) | 16 (32%) | 19 (49%) | 1 (10%) | 0.052 |
| NSAIDS | 3 (3%) | 1 (2%) | 2 (5%) | 0 (0%) | 0.70 |
| Intravenous immunoglobulin | 18 (18%) | 3 (6%) | 15 (38%) | 0 (0%) | <0.001 |
| Plasma exchange | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Immunomodulatory therapy | 15 (15%) | 6 (12%) | 9 (23%) | 0 (0%) | 0.15 |
| Nasogastric feeding or TPN | 12 (12%) | 5 (10%) | 6 (15%) | 0 (0%) | 0.47 |
| Othera | 17 (17%) | 9 (18%) | 7 (18%) | 1 (10%) | 1.00 |
| Antiviral treatment | |||||
| Aciclovir (Intravenous) | 6 (6%) | 0 (0%) | 6 (15%) | 0 (0%) | 0.007 |
| Aciclovir (oral) | 1 (1%) | 0 (0%) | 1 (3%) | 0 (0%) | 0.49 |
| Valaciclovir | 4 (4%) | 2 (4%) | 2 (5%) | 0 (0%) | 1.00 |
| Immunomodulatory therapy | |||||
| Ciclosporin | 3 (3%) | 2 (4%) | 1 (3%) | 0 (0%) | 1.00 |
| Etanercept | 9 (9%) | 1 (2%) | 8 (21%) | 0 (0%) | 0.011 |
| Mepolizumab | 3 (3%) | 3 (6%) | 0 (0%) | 0 (0%) | 0.46 |
| Otherb | 2 (2%) | 2 (4%) | 0 (0%) | 0 (0%) | 0.60 |
| Supportive treatment types | |||||
| Nasogastric feeding | 3 (3%) | 0 (0%) | 3 (8%) | 0 (0%) | 0.13 |
| Parenteral feeding | 2 (2%) | 0 (0%) | 2 (5%) | 0 (0%) | 0.35 |
| Transfusion of blood products | 3 (3%) | 1 (2%) | 2 (5%) | 0 (0%) | 0.70 |
| Type of surgical treatment | |||||
| Skin debridement (ward) | 1 (1%) | 0 (0%) | 1 (3%) | 0 (0%) | 0.49 |
| Skin debridement (theatre) | 14 (14%) | 0 (0%) | 14 (36%) | 0 (0%) | <0.001 |
| Ophthalmic | 2 (2%) | 0 (0%) | 2 (5%) | 0 (0%) | 0.35 |
| None | 83 (83%) | 49 (98%) | 23 (59%) | 10 (100%) | <0.001 |
| Complications from treatment for SCAR | |||||
| Diabetes (including steroid-induced diabetes | 9 (9%) | 4 (8%) | 4 (10%) | 1 (10%) | 0.89 |
| Osteoporosis | 1 (1%) | 0 (0%) | 1 (3%) | 0 (0%) | 0.49 |
| Opportunistic infection | 7 (7%) | 0 (0%) | 7 (18%) | 0 (0%) | 0.003 |
| Death | 3 (3%) | 0 (0%) | 3 (7.7%) | 0 (0%) | 0 (0%) |
| None | 68 (68%) | 32 (64%) | 26 (67%) | 9 (90%) | 0.30 |
| Otherc | 8 (8%) | 5 (10%) | 3 (7.7%) | 0 (0%) | 0.47 |
| Length of stay (at participating site, from admission to discharge) (median,IQR) | 11 (6, 29) (n = 97) | 11 (7, 41) (n = 47) | 14 (7, 27) | 4 (2, 7) | 0.003 |
| Inpatient mortality | 7 (7%) | 0 (0%) | 7 (18%) | 0 (0%) | 0.003 |
| Did the patient die as a result of SCAR? | 4/7 (57%) | 0 (0%) | 4/7 (57%) | 0 (0%) | |
| Readmission 90 days | |||||
| No | 59 (59%) | 27 (54%) | 25 (64%) | 6 (60%) | 0.43 |
| Yes | 16 (16%) | 10 (20%) | 4 (10%) | 2 (20%) | |
| Unknown | 25 (25%) | 13 (26%) | 10 (26%) | 2 (20%) | |
| Readmission with complication of SCAR | 8 (8%) | 4 (8%) | 4 (10%) | 0 (0%) | 0.040 |
| Specialist outpatient follow up in 90 days post discharge? | |||||
| No | 22 (22%) | 8 (16%) | 10 (26%) | 3 (30%) | 0.47 |
| Yes | 67 (67%) | 35 (70%) | 26 (67%) | 6 (60%) | |
| Unknown | 11 (11%) | 7 (14%) | 3 (8%) | 1 (10%) | |
| Skin testing post discharge | |||||
| No | 49 (49%) | 20 (40%) | 24 (62%) | 4 (40%) | 0.039 |
| Yes | 28 (28%) | 18 (36%) | 6 (15%) | 4 (40%) | |
| Unknown | 23 (23%) | 12 (24%) | 9 (23%) | 2 (20%) | |
| Disability at 90 days19 | |||||
| No | 74 (74%) | 36 (72%) | 29 (74%) | 8 (80%) | 0.88 |
| Yes | 8 (8%) | 4 (8%) | 4 (10%) | 0 (0%) | |
| Unknown | 18 (18%) | 10 (20%) | 6 (15%) | 2 (20%) | |
| Mortality 90 days | |||||
| No | 75 (75%) | 40 (80%) | 26 (67%) | 8 (80%) | 0.022 |
| Yes | 11 (11%) | 2 (4%) | 9 (23%) | 0 (0%) | |
| Attributable to SCAR | 7/11 (64%) | 1/2 (50%) | 6/9 (67%) | 0 (0%) | |
| Unknown | 14 (14%) | 8 (16%) | 4 (10%) | 2 (20%) |
Abbreviations: AGEP, acute generalized oedematous pustulosis; SJS, Stevens Johnson Syndrome; TEN, toxic epidermal necrolysis; DRESS, drug reaction with eosinophilia and systemic symptoms; IQR, interquartile range; IV, intravenous; IVIG, intravenous immunoglobulin; TPN, total parental nutrition.
Paraffin ointment (n = 1), antihistamines (n = 3), skin emollient Dermeze/antihistamines (n = 1), opioids (n = 1), prednisolone vaginal suppository (n = 1), cetirizine/liquid paraffin (n = 1), etanercept (n = 4), vasopressor support (n = 1), IV methylprednisolone (n = 1), oral prednisolone/betamethasone ointment/dexamethasone mouthwash/mometasone lotion (n = 1).
Tacrolimus (n = 1), tacrolimus/mycophenolate (n = 1).
Hypertension/renal impairment (n = 1), Non-ST-Elevation Myocardial Infarction (NSTEMI)/ischaemic infarcts (n = 1), unknown (n = 1), hyperpigmentation/hyperhidrosis (n = 1), facial flushing/swelling/redness (n = 1), skin dyspigmentation/hair loss (n = 1), skin bruising (n = 1), abnormal LFT/hepatitis/peripheral neuropathy (n = 1).
Discussion
AUS-SCAR is the first Australasian registry for SCAR, and we report the initial Australian data prior to regional expansion. We have demonstrated the establishment of a robust national coordinated approach to the prospective collection of SCAR phenotypic and immunophenotypic data with paired biorepository sampling. AUS-SCAR is the largest cohort published to-date from the Southern Hemisphere, including First Nations patients from our region (ie, Oceanian). AUS-SCAR lays the foundation for translational and health services research in SCAR to drive changes in prevention, diagnosis and management guidelines and policy.
With regard to phenotypic distribution, AUS-SCAR found a DRESS predominance, which is similar to a recent Thai experience,22 but different to other Asian and European experiences23 and may reflect the variation in clinicians involved, number of burn units currently participating (n = 3), and focus of the sites. Regarding causative drugs, beta-lactam, glycopeptide and sulfonamide antibiotics were most commonly implicated in AUS-SCAR compared to allopurinol, antiepileptics and sulfonamides in Asia and Europe.22, 23, 24, 25, 26, 27 This likely reflects the SJS/TEN predominance in these international cohorts, variance in HLA predisposition, different prescribing patterns and, perhaps most importantly, potential ascertainment bias. Whilst the pharmacogenomic data are not presented in this manuscript, 95% of patients had DNA collected, which will further our understanding of HLA predisposition. Regarding diagnostics, more than 67% of patients had specialist care follow-up, yet only 28% proceeded to skin testing to help ascertain drug causality. Only 38% of cases had a single implicated drug, therefore there is a larger proportion where testing may have aided causality, and even in single drug implicated cases may aid safety of prescribing of class related drugs (eg, flucloxacillin DRESS and safety of cephalosporins). It further highlights the potential deficiencies in SCAR prevention and causality diagnosis.
SCAR phenotypes are associated with significant morbidity. Almost 1 in 4 required ICU admission, median length of stay (LOS) was >10 days and cases occurred in a comorbid population, including the immunocompromised. The mortality associated with SJS/TEN in our cohort closely resembled reports from the USA (14%), Thailand (17%),22 and Korea (15.7%).27,28 However, it was lower than other reported Asian and European experiences (mortality of 35.1% and 28%, respectively).25,29 When comparing to a SCAR dataset from Thailand,22 our overall inpatient mortality was numerically lower (7% vs 9.2%). Future analysis to identify predictors of mortality will be of value, however at present current cohort size is a limitation. In addition to improving mortality metrics, survival morbidity is important and remains understudied.30 AUS-SCAR has planned routine 12-month follow up and QOL assessment;19 the results will drive the development of minimum care standards and a benchmarking framework for clinicians and hospitals.
The current AUS-SCAR does have limitations, many of which we hope to overcome with development of the biorepository and increased site activation and patient recruitment. The current registry includes 4 burns units, with only 3 contributing data to this report. However, recruitment of all Australasian burns centres and multidisciplinary engagement is likely to improve data generalisability and include a greater representation of SJS/TEN cases. Currently, the AUS-SCAR framework remains a repository without an ability to feedback to patients post discharge. We aim to close this loop by conversion of AUS-SCAR to a clinical quality registry, and subsequent development of a minimum care standard for Australasian hospitals. We hope in the future that AUS-SCAR can inform the generation and implementation of such frameworks, provide sites with benchmarking capability and enable a patient facing element to aid access to culprit drug identification and causality testing (in vivo and ex vivo/in vitro). Further, much of the interest is improving drug causality assessment by ex vivo and in vitro work which has been piloted in SCAR,13,20 and recent advancements in artificial intelligence and machine learning algorithm.31 We hope that with a larger sample size international network-of-network collaborations this will also be possible. Nonetheless, the first 100 cases of AUS-SCAR provide the establishment of the registry as a biorepository as well as an insight into SCAR in a varied Southern Hemisphere population that provides a roadmap to focus the research agenda and care provided to SCAR patients.
Abbreviations
AGEP, acute generalized oedematous pustulosis; ATSI, Aboriginal and Torres Strait Islanders; BSA, Body Surface Area; CCI, age-adjusted Charlson Comorbidity Index; DRESS, drug reaction with eosinophilia and systemic symptoms; GBFDE, Generalized bullous fixed drug eruption; HLA, Human Leukocyte Antigen; IV, intravenous; IVIG, intravenous immunoglobulin; IQR, interquartile range; NSAID, non-steroidal anti-inflammatory drug; PPI, proton-pump inhibitors; SCAR, Severe Cutaneous Adverse Reactions; SJS, Stevens Johnson Syndrome; SSRI, selective serotine receptor inhibitors; TEN, toxic epidermal necrolysis; TPN, total parental nutrition.
Funding
This registry was funded by the Allergy and Immunology Foundation of Australasia (AIFA 2019). Prof Trubiano is funded by an NHMRC Emerging Leadership Fellowship (EL2).
Availability of data
Data supporting this study is not available publicly.
Author contributions
Conception and design of the study: Trubiano, Phillips, James, Vogrin.
Acquisition, analysis, or interpretation of data: all authors.
Statistical analysis: Vogrin.
Drafting the article or critical revision of the manuscript for important intellectual consent: all authors.
All authors approved the publication of the manuscript.
Ethics
The project have received ethics approval from the Austin Health Human Research Ethics Committee (Project reference number: HREC/50791/Austin-2019).
Declaration of competing interest
No relevant conflicts of interest to declare.
Acknowledgment
The authors would like to acknowledge the participants, investigators and research coordinators for their contributions to the AUS-SCAR database.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2024.100936.
Contributor Information
Fiona James, Email: fiona.james@austin.org.au.
Jason A. Trubiano, Email: trubianoj@unimelb.edu.au.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Duong T.A., et al. Severe cutaneous adverse reactions to drugs. Lancet. 2017;390(10106):1996–2011. doi: 10.1016/S0140-6736(16)30378-6. [DOI] [PubMed] [Google Scholar]
- 2.Adler N.R., et al. Recent advances in the understanding of severe cutaneous adverse reactions. Br J Dermatol. 2017;177(5):1234–1247. doi: 10.1111/bjd.15423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duong T.A., et al. Severe cutaneous adverse reactions to drugs. Lancet (London, England) 2017;390(10106):1996–2011. doi: 10.1016/S0140-6736(16)30378-6. [DOI] [PubMed] [Google Scholar]
- 4.White K.D., et al. SJS/TEN 2017: building multidisciplinary networks to drive science and translation. J Allergy Clin Immunol Pract. 2018;6(1):38–69. doi: 10.1016/j.jaip.2017.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang W.C., et al. SJS/TEN 2019: from science to translation. J Dermatol Sci. 2020;98(1):2–12. doi: 10.1016/j.jdermsci.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phillips E.J., Mallal S.A. Pharmacogenetics of drug hypersensitivity. Pharmacogenomics. 2010;11(7):973–987. doi: 10.2217/pgs.10.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mallal S., et al. HLA-B∗5701 screening for hypersensitivity to abacavir. N Engl J Med. 2008;358(6):568–579. doi: 10.1056/NEJMoa0706135. [DOI] [PubMed] [Google Scholar]
- 8.Yun J., et al. Oxypurinol directly and immediately activates the drug-specific T cells via the preferential use of HLA-B∗58:01. J Immunol. 2014;192(7):2984–2993. doi: 10.4049/jimmunol.1302306. [DOI] [PubMed] [Google Scholar]
- 9.Chung W.H., et al. Medical genetics: a marker for Stevens-Johnson syndrome. Nature. 2004;428(6982):486. doi: 10.1038/428486a. [DOI] [PubMed] [Google Scholar]
- 10.Konvinse K.C., et al. HLA-A∗32:01 is strongly associated with vancomycin-induced drug reaction with eosinophilia and systemic symptoms. J Allergy Clin Immunol. 2019;144(1):183–192. doi: 10.1016/j.jaci.2019.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trubiano J.A., et al. A comparative analysis between antibiotic- and nonantibiotic-associated delayed cutaneous adverse drug reactions. J Allergy Clin Immunol Pract. 2016;4(6):1187–1193. doi: 10.1016/j.jaip.2016.04.026. [DOI] [PubMed] [Google Scholar]
- 12.Copaescu A.M., Trublano J.A. The assessment of severe cutaneous adverse drug reactions. Aust Prescr. 2022;45(2):43–48. doi: 10.18773/austprescr.2022.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Copaescu A., et al. An updated review of the diagnostic methods in delayed drug hypersensitivity. Front Pharmacol. 2020;11 doi: 10.3389/fphar.2020.573573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trubiano J.A., et al. Return to sender: the need to re-address patient antibiotic allergy labels in Australia and New Zealand. Intern Med J. 2016;46(11):1311–1317. doi: 10.1111/imj.13221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Derrick M.I., et al. A survey of drug allergy training opportunities in the United States. J Allergy Clin Immunol Pract. 2018;6(1):302–304. doi: 10.1016/j.jaip.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phillips E.J., et al. Controversies in drug allergy: testing for delayed reactions. J Allergy Clin Immunol. 2019;143(1):66–73. doi: 10.1016/j.jaci.2018.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kardaun S.H., et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. 2013;169(5):1071–1080. doi: 10.1111/bjd.12501. [DOI] [PubMed] [Google Scholar]
- 18.Rojas Mejía D.V., et al. Severe cutaneous adverse reactions to drugs in Latin America: the RACGRAD study. J Investig Allergol Clin Immunol. 2021;31(4):322–331. doi: 10.18176/jiaci.0497. [DOI] [PubMed] [Google Scholar]
- 19.James F., et al. Study protocol: australasian registry of severe cutaneous adverse reactions (AUS-SCAR) BMJ Open. 2022;12(8) doi: 10.1136/bmjopen-2021-055906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Copaescu A., et al. The role of in vivo and ex vivo diagnostic tools in severe delayed immune-mediated adverse antibiotic drug reactions. J Allergy Clin Immunol Pract. 2021;9(5):2010–2015. doi: 10.1016/j.jaip.2020.12.052. [DOI] [PubMed] [Google Scholar]
- 21.Trubiano J.A., et al. Drug-specific upregulation of CD137 on CD8+ T cells aids in the diagnosis of multiple antibiotic toxic epidermal necrolysis. J Allergy Clin Immunol Pract. 2016;5(3):823–826. doi: 10.1016/j.jaip.2016.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chuenboonngarm N., et al. Key factors predicting the in-hospital mortality of patients with severe cutaneous adverse reactions in Thailand. J Eur Acad Dermatol Venereol. 2023;37(9):1881–1890. doi: 10.1111/jdv.19222. [DOI] [PubMed] [Google Scholar]
- 23.Kang D.Y., et al. A nationwide study of severe cutaneous adverse reactions based on the multicenter registry in Korea. J Allergy Clin Immunol Pract. 2021;9(2):929–936 e7. doi: 10.1016/j.jaip.2020.09.011. [DOI] [PubMed] [Google Scholar]
- 24.Huang P.W., et al. Analysis of severe cutaneous adverse reactions (SCARs) in Taiwan drug-injury relief system: 18-year results. J Formos Med Assoc. 2022;121(8):1397–1405. doi: 10.1016/j.jfma.2021.09.025. [DOI] [PubMed] [Google Scholar]
- 25.Naegele D., et al. Incidence of epidermal necrolysis: results of the German registry. J Invest Dermatol. 2020;140(12):2525–2527. doi: 10.1016/j.jid.2020.03.968. [DOI] [PubMed] [Google Scholar]
- 26.Halevy S., et al. Allopurinol is the most common cause of Stevens-Johnson syndrome and toxic epidermal necrolysis in Europe and Israel. J Am Acad Dermatol. 2008;58(1):25–32. doi: 10.1016/j.jaad.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 27.Micheletti R.G., et al. Stevens-johnson syndrome/toxic epidermal necrolysis: a multicenter retrospective study of 377 adult patients from the United States. J Invest Dermatol. 2018;138(11):2315–2321. doi: 10.1016/j.jid.2018.04.027. [DOI] [PubMed] [Google Scholar]
- 28.Yang M.S., et al. Incidence of stevens-johnson syndrome and toxic epidermal necrolysis: a nationwide population-based study using national health insurance database in Korea. PLoS One. 2016;11(11) doi: 10.1371/journal.pone.0165933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin Y.F., et al. Severe cutaneous adverse reactions related to systemic antibiotics. Clin Infect Dis. 2014;58(10):1377–1385. doi: 10.1093/cid/ciu126. [DOI] [PubMed] [Google Scholar]
- 30.Kano Y., Shiohara T. Long-term outcome of patients with severe cutaneous adverse reactions. Dermatol Sin. 2013;31(4):211–216. [Google Scholar]
- 31.Chongpison Y., et al. IFN-gamma ELISpot-enabled machine learning for culprit drug identification in nonimmediate drug hypersensitivity. J Allergy Clin Immunol. 2024;153(1):193–202. doi: 10.1016/j.jaci.2023.08.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting this study is not available publicly.