Abstract
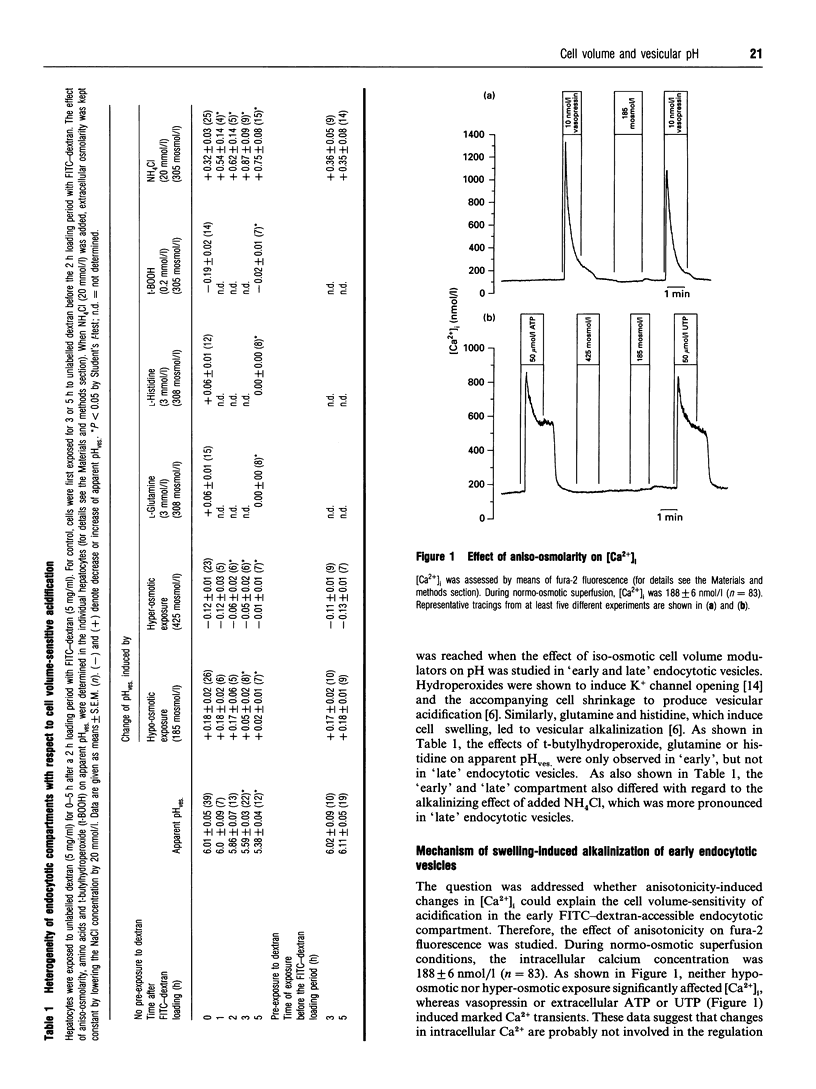
Short-term cultivated rat hepatocytes were allowed to endocytose fluorescein isothiocyanate (FITC)-coupled dextran and the apparent vesicular pH (pHves) was measured by single-cell fluorescence. After 2 h of exposure to FITC-dextran, the apparent pH in the vesicular compartments accessible to endocytosed FITC-dextran was 6.01 +/- 0.05 (n = 39) in normo-osmotic media. Hypo-osmotic exposure increased, whereas hyper-osmotic exposure decreased apparent pHves. by 0.18 +/- 0.02 (n = 26) and 0.12 +/- 0.01 (n = 23) respectively. Incubation of the cells with unlabelled dextran for 2h before a 2-h FITC-dextran exposure had no effect on apparent pHves and its osmosensitivity. When, however, hepatocytes were exposed to unlabelled dextran for 5 h after a 2 h exposure to FITC-dextran, in order to allow transport of endocytosed FITC-dextran to late endocytotic/lysosomal compartments, apparent pHves. decreased to 5.38 +/- 0.04 (n = 12) and the apparent pH in the vesicular compartment containing the dye was no longer sensitive to aniso-osmotic exposure. These findings indicate that the osomosensitivity of pHves. is apparently restricted to early endocytotic compartments. Aniso-osmotic regulation of apparent pHves. in freshly FITC-loaded hepatocytes was not accompanied by aniso-osmolarity-induced changes of the cytosolic free calcium concentration, and neither vasopressin nor extracellular ATP, which provoked a marked Ca2+ signal, affected apparent pHves. Dibutyryl-cyclic AMP (cAMP) or vanadate (0.5 mmol/l) were without effect on apparent pHves. and its osmosensitivity. However, pertussis toxin-treatment or genistein (but not daidzein) or the erbstatin analogue methyl 2,5-dihydroxycinnamate fully abolished the osmo-sensitivity of apparent pHves., but did not affect apparent pHves. It is concluded that regulation of pHves. by cell volume occurs in early endocytotic compartments, but probably not in lysosomes, and is mediated by a G-protein and tyrosine kinase-dependent, but Ca2+- and cAMP-independent mechanism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anbari M., Root K. V., Van Dyke R. W. Role of Na,K-ATPase in regulating acidification of early rat liver endocytic vesicles. Hepatology. 1994 Apr;19(4):1034–1043. [PubMed] [Google Scholar]
- Aplin A., Jasionowski T., Tuttle D. L., Lenk S. E., Dunn W. A., Jr Cytoskeletal elements are required for the formation and maturation of autophagic vacuoles. J Cell Physiol. 1992 Sep;152(3):458–466. doi: 10.1002/jcp.1041520304. [DOI] [PubMed] [Google Scholar]
- Arai H., Pink S., Forgac M. Interaction of anions and ATP with the coated vesicle proton pump. Biochemistry. 1989 Apr 4;28(7):3075–3082. doi: 10.1021/bi00433a051. [DOI] [PubMed] [Google Scholar]
- Bae H. R., Verkman A. S. Protein kinase A regulates chloride conductance in endocytic vesicles from proximal tubule. Nature. 1990 Dec 13;348(6302):637–639. doi: 10.1038/348637a0. [DOI] [PubMed] [Google Scholar]
- Baquet A., Meijer A. J., Hue L. Hepatocyte swelling increases inositol 1,4,5-trisphosphate, calcium and cyclic AMP concentration but antagonizes phosphorylase activation by Ca2(+)-dependent hormones. FEBS Lett. 1991 Jan 14;278(1):103–106. doi: 10.1016/0014-5793(91)80094-j. [DOI] [PubMed] [Google Scholar]
- Barasch J., Kiss B., Prince A., Saiman L., Gruenert D., al-Awqati Q. Defective acidification of intracellular organelles in cystic fibrosis. Nature. 1991 Jul 4;352(6330):70–73. doi: 10.1038/352070a0. [DOI] [PubMed] [Google Scholar]
- Bishop W. R., Petrin J., Wang L., Ramesh U., Doll R. J. Inhibition of protein kinase C by the tyrosine kinase inhibitor erbstatin. Biochem Pharmacol. 1990 Nov 1;40(9):2129–2135. doi: 10.1016/0006-2952(90)90245-g. [DOI] [PubMed] [Google Scholar]
- Busch G. L., Schreiber R., Dartsch P. C., Völkl H., Vom Dahl S., Häussinger D., Lang F. Involvement of microtubules in the link between cell volume and pH of acidic cellular compartments in rat and human hepatocytes. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):9165–9169. doi: 10.1073/pnas.91.19.9165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgac M. Structure and function of vacuolar class of ATP-driven proton pumps. Physiol Rev. 1989 Jul;69(3):765–796. doi: 10.1152/physrev.1989.69.3.765. [DOI] [PubMed] [Google Scholar]
- Galcheva-Gargova Z., Dérijard B., Wu I. H., Davis R. J. An osmosensing signal transduction pathway in mammalian cells. Science. 1994 Aug 5;265(5173):806–808. doi: 10.1126/science.8047888. [DOI] [PubMed] [Google Scholar]
- Grantham J. J. The renal sodium pump and vanadate. Am J Physiol. 1980 Aug;239(2):F97–106. doi: 10.1152/ajprenal.1980.239.2.F97. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hallbrucker C., Ritter M., Lang F., Gerok W., Häussinger D. Hydroperoxide metabolism in rat liver. K+ channel activation, cell volume changes and eicosanoid formation. Eur J Biochem. 1993 Feb 1;211(3):449–458. doi: 10.1111/j.1432-1033.1993.tb17570.x. [DOI] [PubMed] [Google Scholar]
- Han J., Lee J. D., Bibbs L., Ulevitch R. J. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994 Aug 5;265(5173):808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- Häussinger D., Lang F., Gerok W. Regulation of cell function by the cellular hydration state. Am J Physiol. 1994 Sep;267(3 Pt 1):E343–E355. doi: 10.1152/ajpendo.1994.267.3.E343. [DOI] [PubMed] [Google Scholar]
- Häussinger D., Saha N., Hallbrucker C., Lang F., Gerok W. Involvement of microtubules in the swelling-induced stimulation of transcellular taurocholate transport in perfused rat liver. Biochem J. 1993 Apr 15;291(Pt 2):355–360. doi: 10.1042/bj2910355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häussinger D., Stoll B., vom Dahl S., Theodoropoulos P. A., Markogiannakis E., Gravanis A., Lang F., Stournaras C. Effect of hepatocyte swelling on microtubule stability and tubulin mRNA levels. Biochem Cell Biol. 1994 Jan-Feb;72(1-2):12–19. doi: 10.1139/o94-003. [DOI] [PubMed] [Google Scholar]
- Lake J. R., Licko V., Van Dyke R. W., Scharschmidt B. F. Biliary secretion of fluid-phase markers by the isolated perfused rat liver. Role of transcellular vesicular transport. J Clin Invest. 1985 Aug;76(2):676–684. doi: 10.1172/JCI112021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake J. R., Van Dyke R. W., Scharschmidt B. F. Acidic vesicles in cultured rat hepatocytes. Identification and characterization of their relationship to lysosomes and other storage vesicles. Gastroenterology. 1987 May;92(5 Pt 1):1251–1261. [PubMed] [Google Scholar]
- Meijer A. J., Gimpel J. A., Deleeuw G. A., Tager J. M., Williamson J. R. Role of anion translocation across the mitochondrial membrane in the regulation of urea synthesis from ammonia by isolated rat hepatocytes. J Biol Chem. 1975 Oct 10;250(19):7728–7738. [PubMed] [Google Scholar]
- Mellman I., Fuchs R., Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- Mulberg A. E., Tulk B. M., Forgac M. Modulation of coated vesicle chloride channel activity and acidification by reversible protein kinase A-dependent phosphorylation. J Biol Chem. 1991 Nov 5;266(31):20590–20593. [PubMed] [Google Scholar]
- Murphy R. F. Analysis and isolation of endocytic vesicles by flow cytometry and sorting: demonstration of three kinetically distinct compartments involved in fluid-phase endocytosis. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8523–8526. doi: 10.1073/pnas.82.24.8523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasenick M. M., Wang N., Yan K. Specific associations between tubulin and G proteins: participation of cytoskeletal elements in cellular signal transduction. Adv Second Messenger Phosphoprotein Res. 1990;24:381–386. [PubMed] [Google Scholar]
- Rindress D., Lei X., Ahluwalia J. P., Cameron P. H., Fazel A., Posner B. I., Bergeron J. J. Organelle-specific phosphorylation. Identification of unique membrane phosphoproteins of the endoplasmic reticulum and endosomal apparatus. J Biol Chem. 1993 Mar 5;268(7):5139–5147. [PubMed] [Google Scholar]
- Rouse J., Cohen P., Trigon S., Morange M., Alonso-Llamazares A., Zamanillo D., Hunt T., Nebreda A. R. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994 Sep 23;78(6):1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- Sarkadi B., Parker J. C. Activation of ion transport pathways by changes in cell volume. Biochim Biophys Acta. 1991 Dec 12;1071(4):407–427. doi: 10.1016/0304-4157(91)90005-h. [DOI] [PubMed] [Google Scholar]
- Schliess F., Schreiber R., Häussinger D. Activation of extracellular signal-regulated kinases Erk-1 and Erk-2 by cell swelling in H4IIE hepatoma cells. Biochem J. 1995 Jul 1;309(Pt 1):13–17. doi: 10.1042/bj3090013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R., Stoll B., Lang F., Häussinger D. Effects of aniso-osmolarity and hydroperoxides on intracellular pH in isolated rat hepatocytes as assessed by (2',7')-bis(carboxyethyl)-5(6)-carboxyfluorescein and fluorescein isothiocyanate-dextran fluorescence. Biochem J. 1994 Oct 1;303(Pt 1):113–120. doi: 10.1042/bj3030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strømhaug P. E., Seglen P. O. Evidence for acidity of prelysosomal autophagic/endocytic vacuoles (amphisomes). Biochem J. 1993 Apr 1;291(Pt 1):115–121. doi: 10.1042/bj2910115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979 May 29;18(11):2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Umezawa K., Hori T., Tajima H., Imoto M., Isshiki K., Takeuchi T. Inhibition of epidermal growth factor-induced DNA synthesis by tyrosine kinase inhibitors. FEBS Lett. 1990 Jan 29;260(2):198–200. doi: 10.1016/0014-5793(90)80102-o. [DOI] [PubMed] [Google Scholar]
- Van Dyke R. W. Acidification of rat liver lysosomes: quantitation and comparison with endosomes. Am J Physiol. 1993 Oct;265(4 Pt 1):C901–C917. doi: 10.1152/ajpcell.1993.265.4.C901. [DOI] [PubMed] [Google Scholar]
- Van Dyke R. W., Root K. V. Ethinyl estradiol decreases acidification of rat liver endocytic vesicles. Hepatology. 1993 Sep;18(3):604–613. [PubMed] [Google Scholar]
- Völkl H., Busch G. L., Häussinger D., Lang F. Alkalinization of acidic cellular compartments following cell swelling. FEBS Lett. 1994 Jan 24;338(1):27–30. doi: 10.1016/0014-5793(94)80110-x. [DOI] [PubMed] [Google Scholar]
- Völkl H., Friedrich F., Häussinger D., Lang F. Effect of cell volume on Acridine Orange fluorescence in hepatocytes. Biochem J. 1993 Oct 1;295(Pt 1):11–14. doi: 10.1042/bj2950011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S. W., Poole R. C., Hudson A. T., Halestrap A. P., Denton R. M., Tavaré J. M. Effects of tyrosine kinase inhibitors on protein kinase-independent systems. FEBS Lett. 1993 Feb 1;316(3):278–282. doi: 10.1016/0014-5793(93)81308-m. [DOI] [PubMed] [Google Scholar]
- de Duve C. Lysosomes revisited. Eur J Biochem. 1983 Dec 15;137(3):391–397. doi: 10.1111/j.1432-1033.1983.tb07841.x. [DOI] [PubMed] [Google Scholar]


