Abstract
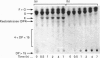
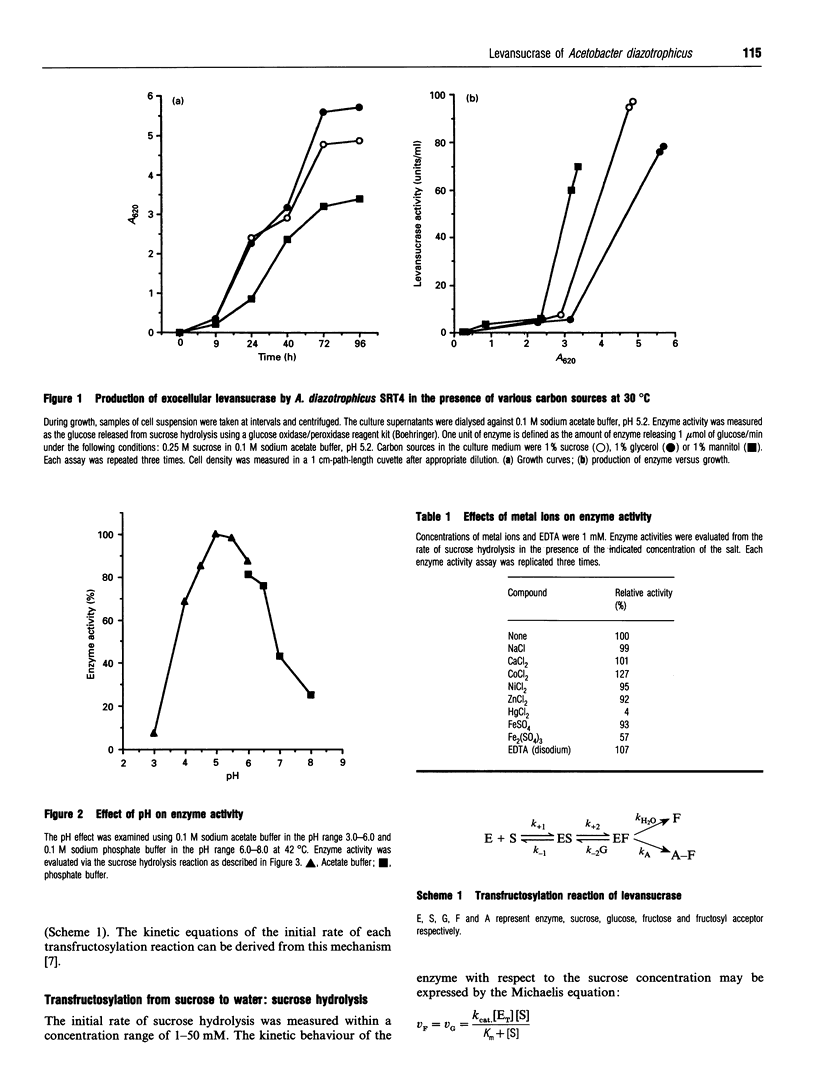
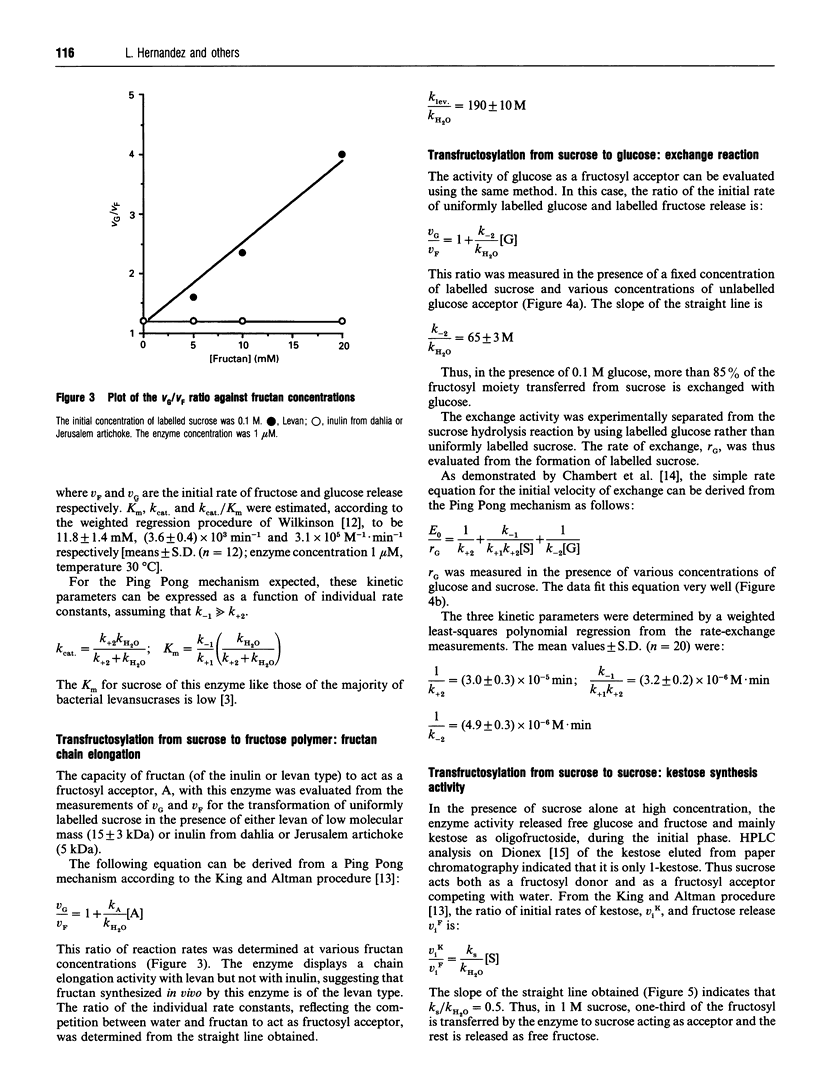
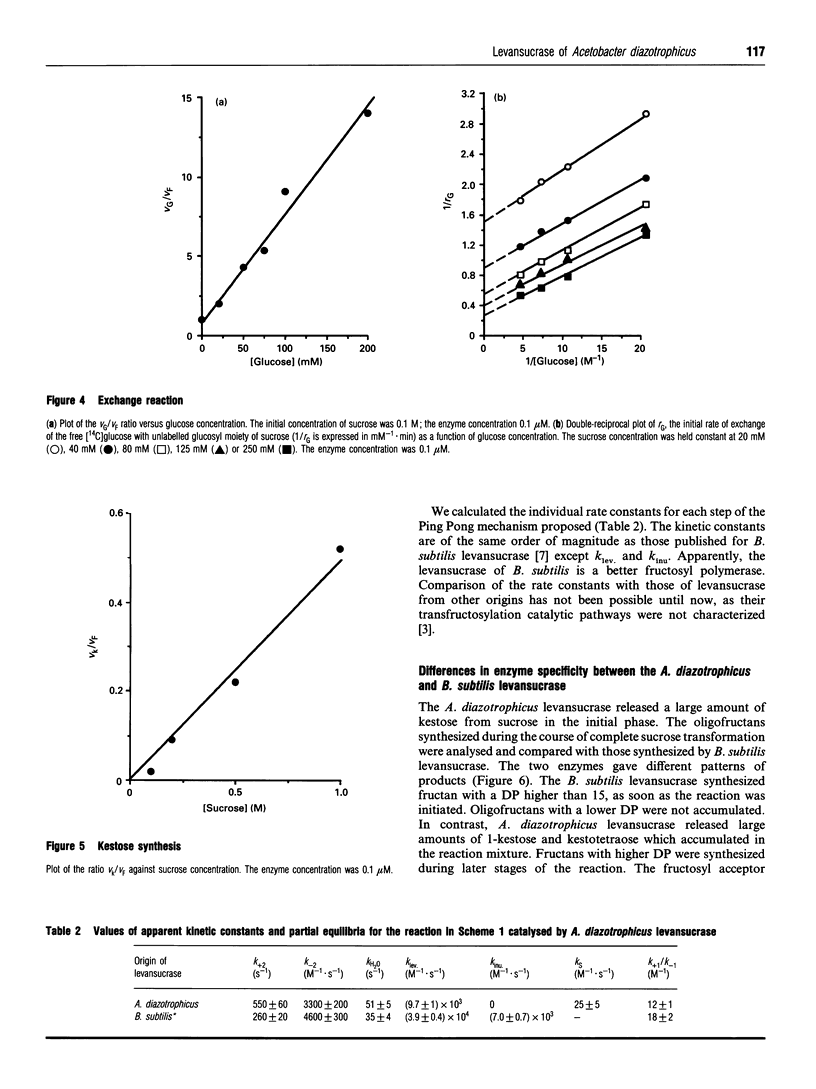
Acetobacter diazotrophicus, a nitrogen-fixing bacterium associated with sugar cane, secretes a levansucrase (sucrose-2,6-beta-D-fructan 6-beta-D-fructosyltransferase; EC 2.4.1.10). This enzyme is constitutively expressed and represents more than 70% of the total proteins secreted by strain SRT4. The purified protein consists of a single 58 kDa polypeptide with an isoelectric point of 5.5. Its activity is optimal at pH 5.0. It catalyses transfructosylation from sucrose to a variety of acceptors including water (sucrose hydrolysis), glucose (exchange reaction), fructan (polymerase reaction) and sucrose (oligofructoside synthesis). In vivo the polymerase activity leads to synthesis of a high-molecular-mass fructan of the levan type. A. diazotrophicus levansucrase catalyses transfructosylation via a Ping Pong mechanism involving the formation of a transient fructosyl-enzyme intermediate. The catalytic mechanism is very similar to that of Bacillus subtilis levansucrase. The kinetic parameters of the two enzymes are of the same order of magnitude. The main difference between the two enzyme specificities is the high yield of oligofructoside, particularly 1-kestotriose and kestotetraose, accumulated by A. diazotrophicus levansucrase during sucrose transformation. We discuss the hypothesis that these catalytic features may serve the different biological functions of each enzyme.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chambert R., Gonzy-Tréboul G. Levansucrase of Bacillus subtilis: kinetic and thermodynamic aspects of transfructosylation processes. Eur J Biochem. 1976 Feb 2;62(1):55–64. doi: 10.1111/j.1432-1033.1976.tb10097.x. [DOI] [PubMed] [Google Scholar]
- Chambert R., Petit-Glatron M. F. Polymerase and hydrolase activities of Bacillus subtilis levansucrase can be separately modulated by site-directed mutagenesis. Biochem J. 1991 Oct 1;279(Pt 1):35–41. doi: 10.1042/bj2790035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambert R., Treboul G., Dedonder R. Kinetic studies of levansucrase of Bacillus subtilis. Eur J Biochem. 1974 Jan 16;41(2):285–300. doi: 10.1111/j.1432-1033.1974.tb03269.x. [DOI] [PubMed] [Google Scholar]
- Chen J., Baithun S. I., Pollock D. J., Berry C. L. Argyrophilic and hormone immunoreactive cells in normal and hyperplastic pancreatic ducts and exocrine pancreatic carcinoma. Virchows Arch A Pathol Anat Histopathol. 1988;413(5):399–405. doi: 10.1007/BF00716988. [DOI] [PubMed] [Google Scholar]
- Fernandez-Patron C., Castellanos-Serra L., Rodriguez P. Reverse staining of sodium dodecyl sulfate polyacrylamide gels by imidazole-zinc salts: sensitive detection of unmodified proteins. Biotechniques. 1992 Apr;12(4):564–573. [PubMed] [Google Scholar]
- Leigh J. A., Coplin D. L. Exopolysaccharides in plant-bacterial interactions. Annu Rev Microbiol. 1992;46:307–346. doi: 10.1146/annurev.mi.46.100192.001515. [DOI] [PubMed] [Google Scholar]
- Russell R. R. Use of triton X-100 to overcome the inhibition of fructosyltransferase by SDS. Anal Biochem. 1979 Aug;97(1):173–175. doi: 10.1016/0003-2697(79)90342-7. [DOI] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]