Abstract
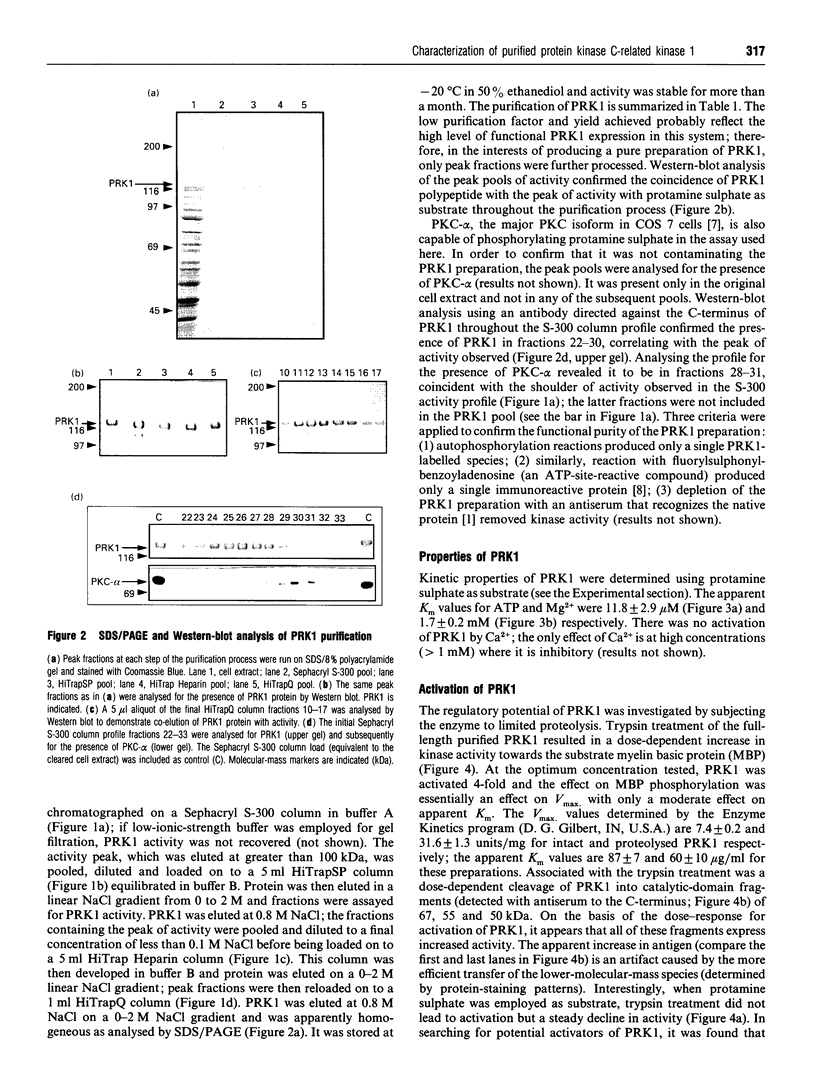
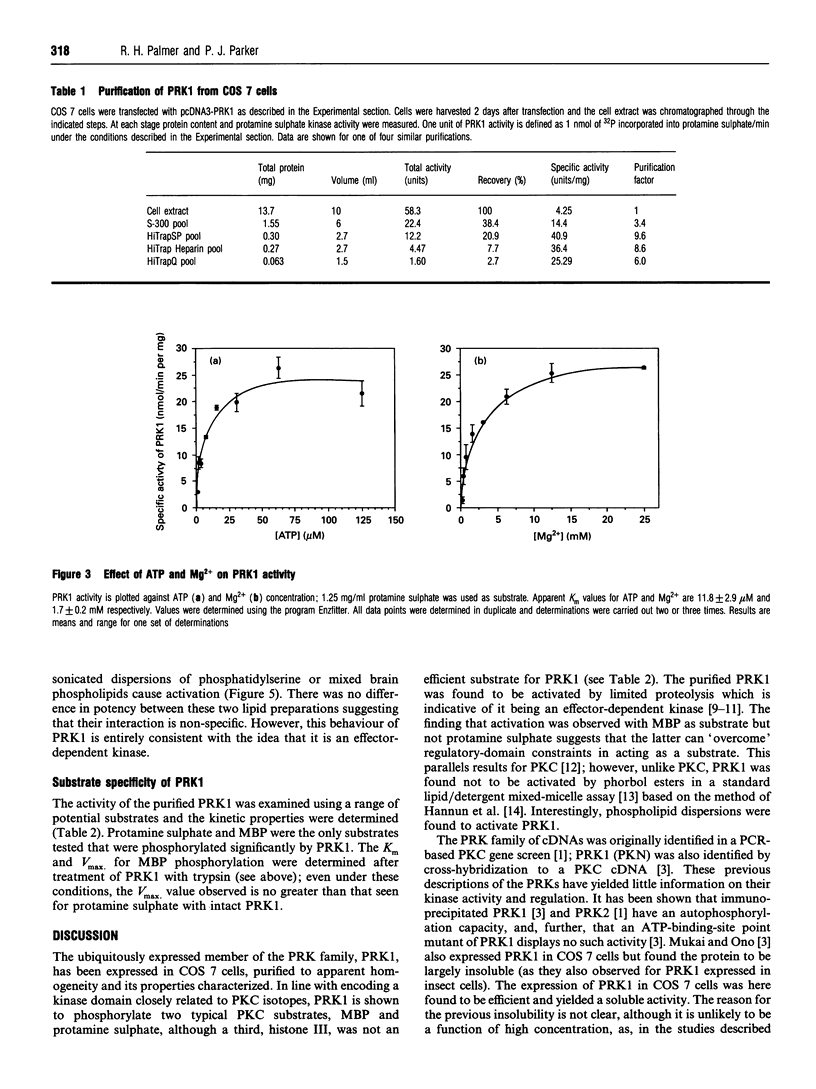
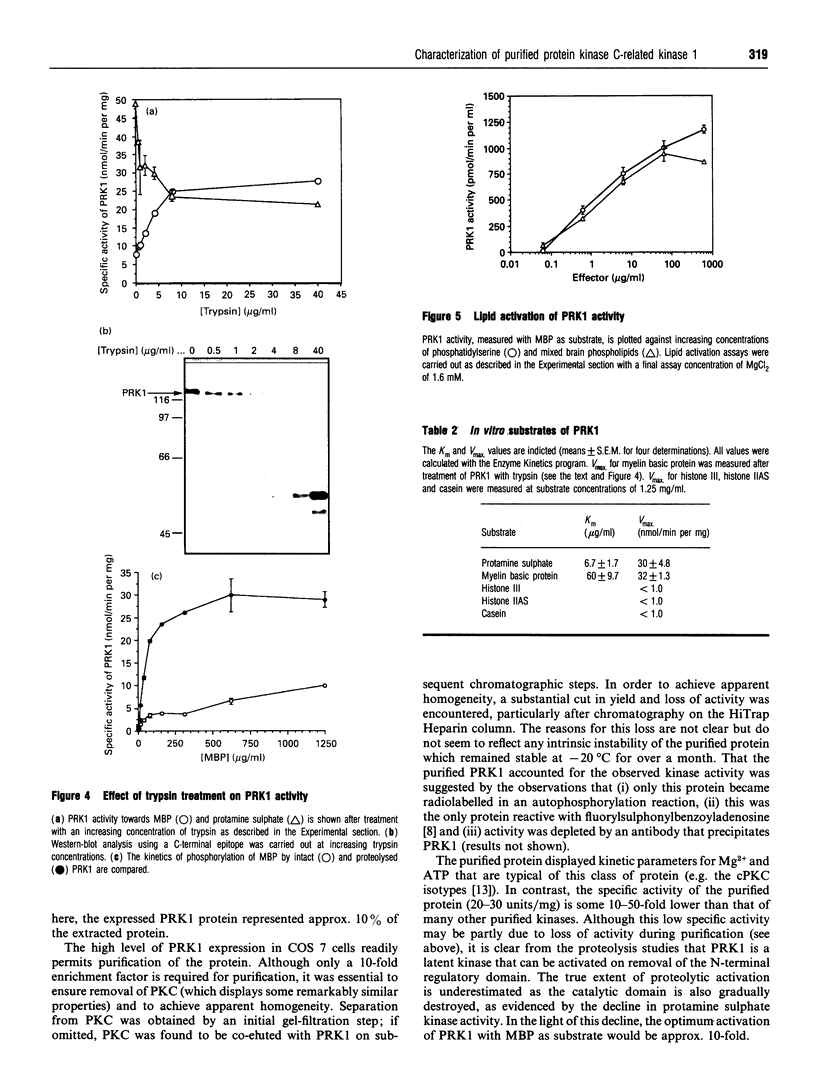
The recently described protein kinase C-related kinase (PRK) family is comprised of at least three members: PRK1, PRK2 and PRK3. Here the expression, purification and characterization of the ubiquitously expressed isoform, PRK1, is described. The enzyme was expressed in COS 7 cells and subsequently purified to apparent homogeneity by sequential column chromatography. The purified PRK1 protein migrates as a single 120 kDa polypeptide on SDS/PAGE. It displays a substrate specificity that in part resembles that of protein kinase C (PKC); however, unlike PKC, it is not activated by any combination of phorbol esters, diacylglycerol and Ca2+. Nevertheless, it can be activated by limited proteolysis, indicating a negative regulatory role for the N-terminal domain(s). PRK1 is also activated by phospholipids. The physiological relevance of this activation is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazzi M. D., Nelsestuen G. L. Substrate-specific stimulation of protein kinase C by polyvalent anion. Biochem Biophys Res Commun. 1987 Aug 31;147(1):248–253. doi: 10.1016/s0006-291x(87)80113-4. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Dekker L. V., Parker P. J. Protein kinase C--a question of specificity. Trends Biochem Sci. 1994 Feb;19(2):73–77. doi: 10.1016/0968-0004(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Hannun Y. A., Loomis C. R., Bell R. M. Activation of protein kinase C by Triton X-100 mixed micelles containing diacylglycerol and phosphatidylserine. J Biol Chem. 1985 Aug 25;260(18):10039–10043. [PubMed] [Google Scholar]
- Ikebe M., Stepinska M., Kemp B. E., Means A. R., Hartshorne D. J. Proteolysis of smooth muscle myosin light chain kinase. Formation of inactive and calmodulin-independent fragments. J Biol Chem. 1987 Oct 5;262(28):13828–13834. [PubMed] [Google Scholar]
- Inoue M., Kishimoto A., Takai Y., Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. II. Proenzyme and its activation by calcium-dependent protease from rat brain. J Biol Chem. 1977 Nov 10;252(21):7610–7616. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marais R. M., Parker P. J. Purification and characterisation of bovine brain protein kinase C isotypes alpha, beta and gamma. Eur J Biochem. 1989 Jun 1;182(1):129–137. doi: 10.1111/j.1432-1033.1989.tb14809.x. [DOI] [PubMed] [Google Scholar]
- Morrice N. A., Fecondo J., Wettenhall R. E. Differential effects of fatty acid and phospholipid activators on the catalytic activities of a structurally novel protein kinase from rat liver. FEBS Lett. 1994 Sep 5;351(2):171–175. doi: 10.1016/0014-5793(94)00854-x. [DOI] [PubMed] [Google Scholar]
- Mukai H., Ono Y. A novel protein kinase with leucine zipper-like sequences: its catalytic domain is highly homologous to that of protein kinase C. Biochem Biophys Res Commun. 1994 Mar 15;199(2):897–904. doi: 10.1006/bbrc.1994.1313. [DOI] [PubMed] [Google Scholar]
- Palmer R. H., Ridden J., Parker P. J. Cloning and expression patterns of two members of a novel protein-kinase-C-related kinase family. Eur J Biochem. 1995 Jan 15;227(1-2):344–351. doi: 10.1111/j.1432-1033.1995.tb20395.x. [DOI] [PubMed] [Google Scholar]
- Parker P. J. Antibodies to fluorylsulfonylbenzoyladenosine permit identification of protein kinases. FEBS Lett. 1993 Nov 22;334(3):347–350. doi: 10.1016/0014-5793(93)80709-4. [DOI] [PubMed] [Google Scholar]
- Pears C., Schaap D., Parker P. J. The regulatory domain of protein kinase C-epsilon restricts the catalytic-domain-specificity. Biochem J. 1991 May 15;276(Pt 1):257–260. doi: 10.1042/bj2760257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Inoue M., Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. I. Purification and characterization of an active enzyme from bovine cerebellum. J Biol Chem. 1977 Nov 10;252(21):7603–7609. [PubMed] [Google Scholar]




