Abstract
Cellular signalling by the inflammatory cytokine tumour necrosis factor alpha (TNF alpha) has been suggested to involve generation of low levels of reactive oxygen species (ROS). Certain antioxidants and metal chelators can inhibit cytotoxicity and gene expression in response to TNF alpha in numerous cell types. However, neither the source nor function of TNF alpha-induced oxidant generation is known. Using specific inhibitors, we ruled out involvement of several oxidant-generating enzymes [cyclo-oxygenase (indomethacin), cytochrome P-450 (metyrapone), nitric oxide synthase (NG-methyl-L-arginine), NADPH oxidase (iodonium diphenyl), xanthine oxidase (allopurinol), ribonucleotide reductase (hydroxyurea)] in TNF alpha-mediated apoptosis of the murine fibrosarcoma line, L929. We also demonstrated no role for mitochondrial-derived radicals/respiratory chain in the lytic pathway using specific inhibitors/uncouplers (rotenone, KCN, carboxin, fluoroacetate, antimycin, malonate, carbonyl cyanide p-trifluoromethoxyphenylhydrazone) and chloramphenicol-derived respiration-deficient cells. Significant ROS (H2O2, O2-.) generation was not observed in response to TNF alpha in L929 cells using four separate assays. Also, prevention of intracellular H2O2 removal by inhibition of catalase did not potentiate TNF alpha-mediated cell death. These data suggest that neither H2O2 nor O2-. plays a direct role in TNF alpha cytotoxicity. Finally, we suggest a central role for lipoxygenase in TNF alpha-mediated lysis. Three inhibitors of this radical-generating signalling pathway, including an arachidonate analogue (5,8,11,14-eicosatetraynoic acid), could protect cells against TNF alpha. The inhibitor nordihydroguaiaretic acid is also a radical scavenger, but it could not protect cells from ROS toxicity at concentrations that effectively prevented TNF alpha killing. Therefore protection by nordihydroguaiaretic acid cannot be due to scavenging of cytotoxic H2O or O2-.. The lipoxygenase product, (12S)-hydroxyeicosatetraenoic acid, was also significantly protective. As this analogue can act as a substrate for certain lipoxygenases, this effect may be due to prevention of generation of physiological products.
Full text
PDF

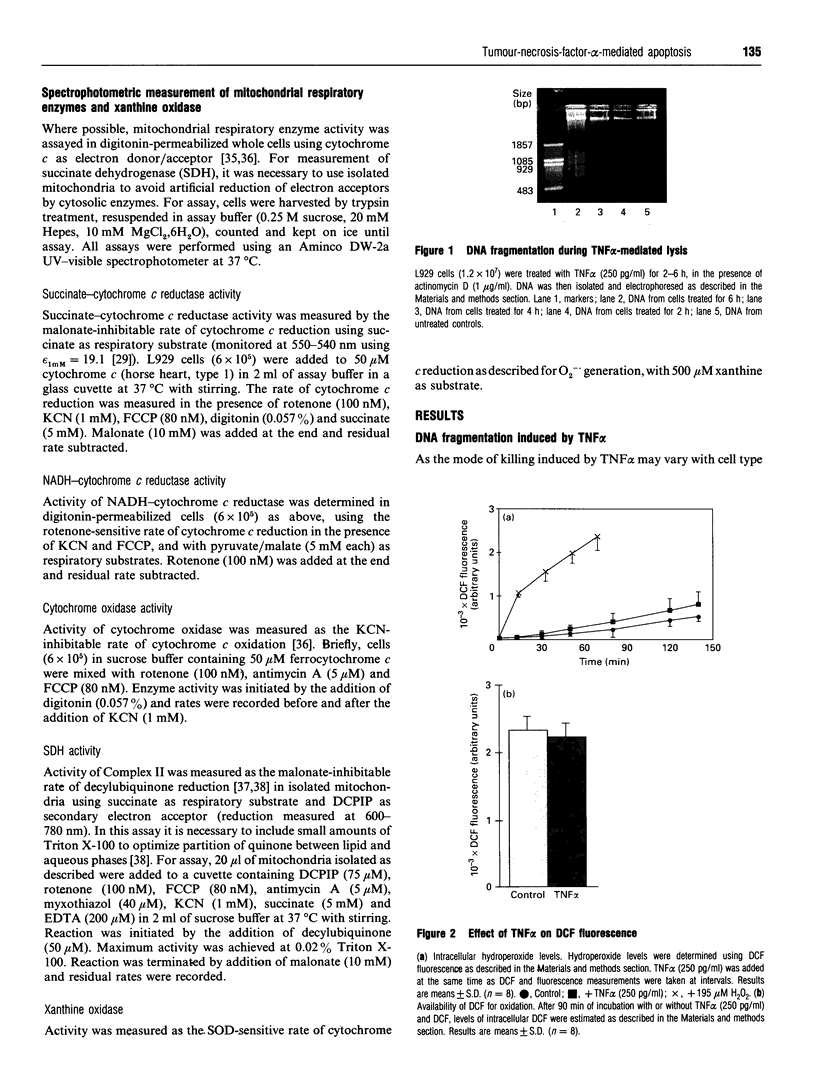
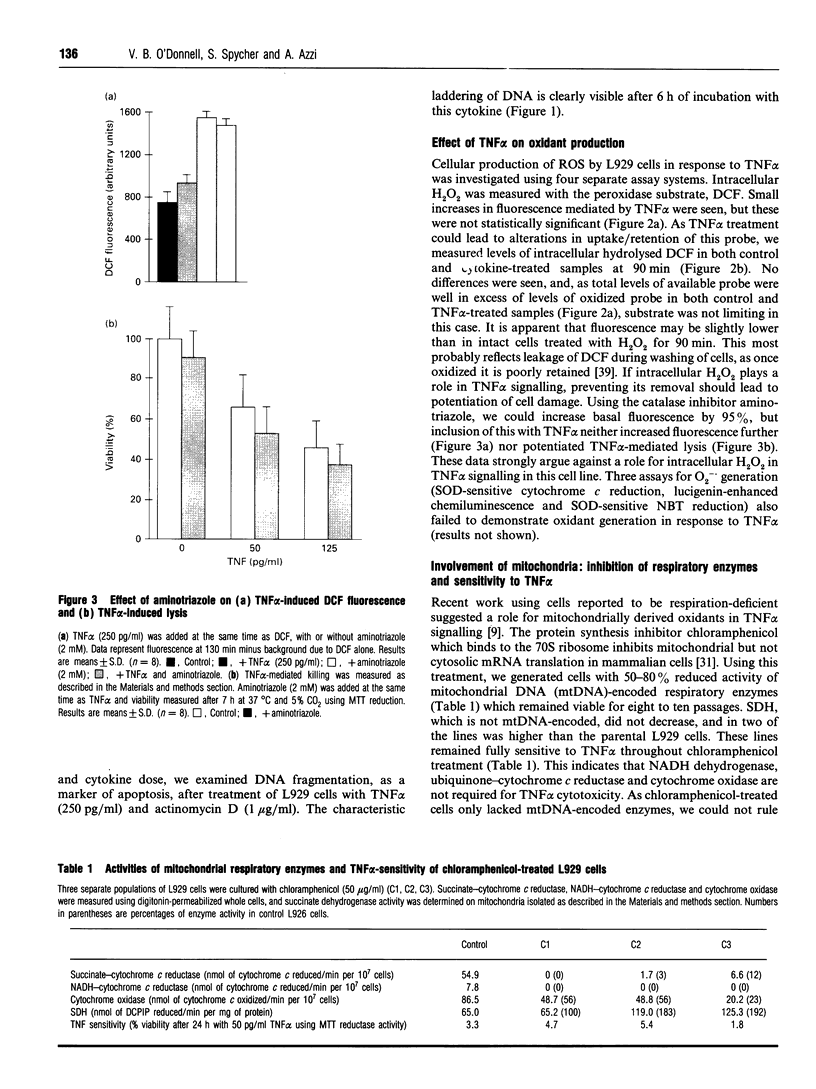
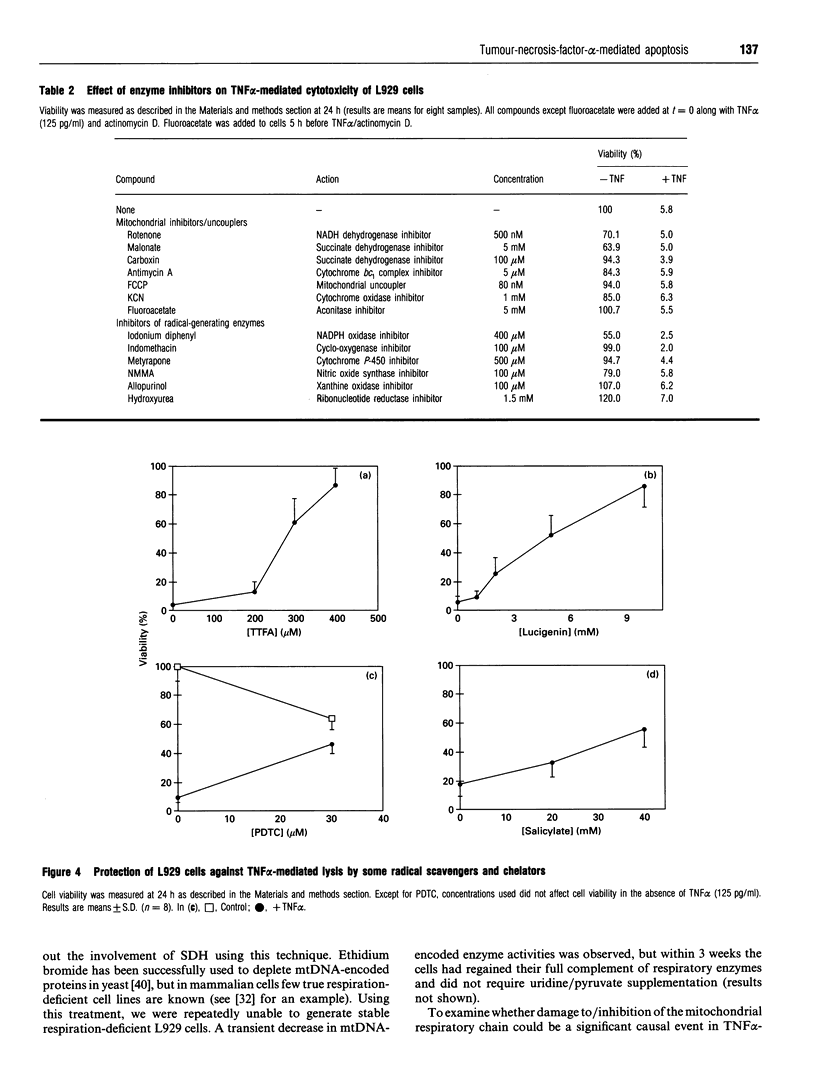
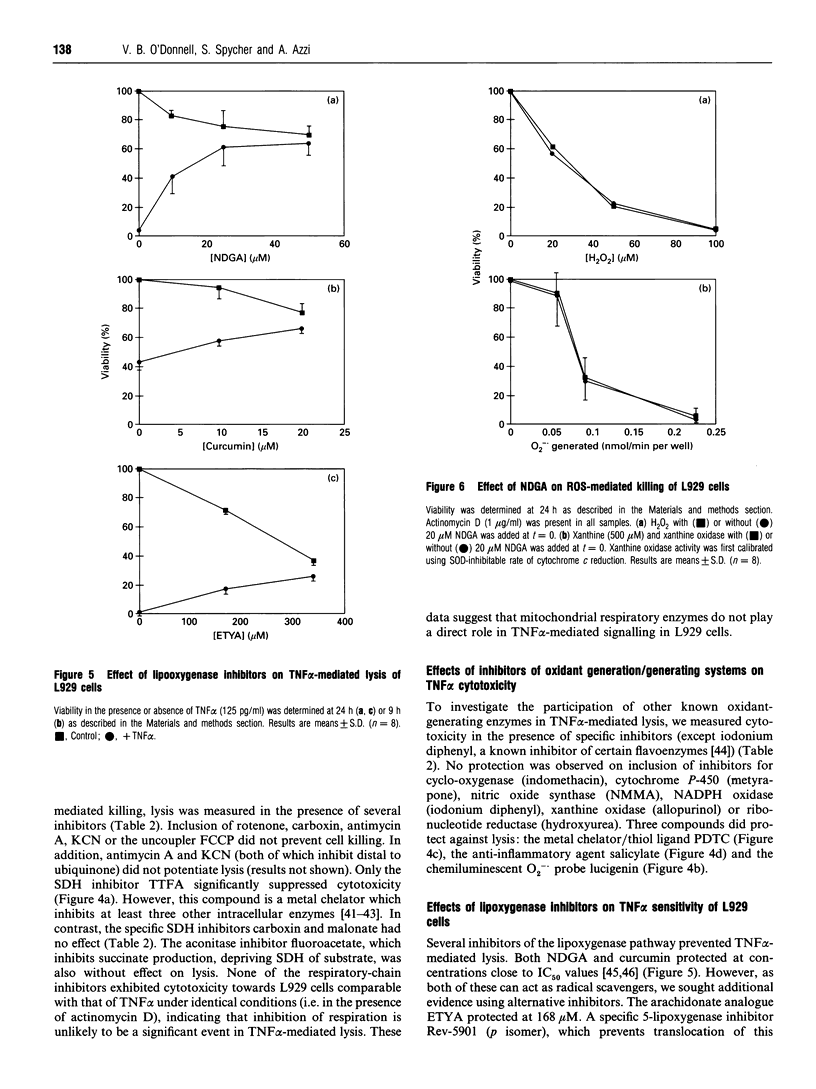


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barradas M. A., Jeremy J. Y., Kontoghiorghes G. J., Mikhailidis D. P., Hoffbrand A. V., Dandona P. Iron chelators inhibit human platelet aggregation, thromboxane A2 synthesis and lipoxygenase activity. FEBS Lett. 1989 Mar 13;245(1-2):105–109. doi: 10.1016/0014-5793(89)80201-7. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brekke O. L., Shalaby M. R., Sundan A., Espevik T., Bjerve K. S. Butylated hydroxyanisole specifically inhibits tumor necrosis factor-induced cytotoxicity and growth enhancement. Cytokine. 1992 Jul;4(4):269–280. doi: 10.1016/1043-4666(92)90067-2. [DOI] [PubMed] [Google Scholar]
- Buttke T. M., Sandstrom P. A. Oxidative stress as a mediator of apoptosis. Immunol Today. 1994 Jan;15(1):7–10. doi: 10.1016/0167-5699(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Cathcart R., Schwiers E., Ames B. N. Detection of picomole levels of hydroperoxides using a fluorescent dichlorofluorescein assay. Anal Biochem. 1983 Oct 1;134(1):111–116. doi: 10.1016/0003-2697(83)90270-1. [DOI] [PubMed] [Google Scholar]
- Chamulitrat W., Hughes M. F., Eling T. E., Mason R. P. Superoxide and peroxyl radical generation from the reduction of polyunsaturated fatty acid hydroperoxides by soybean lipoxygenase. Arch Biochem Biophys. 1991 Oct;290(1):153–159. doi: 10.1016/0003-9861(91)90601-e. [DOI] [PubMed] [Google Scholar]
- Chang D. J., Ringold G. M., Heller R. A. Cell killing and induction of manganous superoxide dismutase by tumor necrosis factor-alpha is mediated by lipoxygenase metabolites of arachidonic acid. Biochem Biophys Res Commun. 1992 Oct 30;188(2):538–546. doi: 10.1016/0006-291x(92)91089-9. [DOI] [PubMed] [Google Scholar]
- Cross A. R., Jones O. T. Enzymic mechanisms of superoxide production. Biochim Biophys Acta. 1991 May 6;1057(3):281–298. doi: 10.1016/s0005-2728(05)80140-9. [DOI] [PubMed] [Google Scholar]
- Dileepan K. N., Kennedy J. Complete inhibition of dihydro-orotate oxidation and superoxide production by 1,1,1-trifluoro-3-thenoylacetone in rat liver mitochondria. Biochem J. 1985 Jan 1;225(1):189–194. doi: 10.1042/bj2250189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias L., Berry C. O. Induction of differentiation by tumour necrosis factor in HL-60 cells is associated with the formation of large DNA fragments. Leukemia. 1991 Oct;5(10):879–885. [PubMed] [Google Scholar]
- Estornell E., Fato R., Pallotti F., Lenaz G. Assay conditions for the mitochondrial NADH:coenzyme Q oxidoreductase. FEBS Lett. 1993 Oct 11;332(1-2):127–131. doi: 10.1016/0014-5793(93)80498-j. [DOI] [PubMed] [Google Scholar]
- Fehsel K., Kolb-Bachofen V., Kolb H. Analysis of TNF alpha-induced DNA strand breaks at the single cell level. Am J Pathol. 1991 Aug;139(2):251–254. [PMC free article] [PubMed] [Google Scholar]
- Fiers W. Tumor necrosis factor. Characterization at the molecular, cellular and in vivo level. FEBS Lett. 1991 Jul 22;285(2):199–212. doi: 10.1016/0014-5793(91)80803-b. [DOI] [PubMed] [Google Scholar]
- Flynn D. L., Rafferty M. F., Boctor A. M. Inhibition of 5-hydroxy-eicosatetraenoic acid (5-HETE) formation in intact human neutrophils by naturally-occurring diarylheptanoids: inhibitory activities of curcuminoids and yakuchinones. Prostaglandins Leukot Med. 1986 Jun;22(3):357–360. doi: 10.1016/0262-1746(86)90146-0. [DOI] [PubMed] [Google Scholar]
- Ford-Hutchinson A. W., Gresser M., Young R. N. 5-Lipoxygenase. Annu Rev Biochem. 1994;63:383–417. doi: 10.1146/annurev.bi.63.070194.002123. [DOI] [PubMed] [Google Scholar]
- Granger D. L., Lehninger A. L. Sites of inhibition of mitochondrial electron transport in macrophage-injured neoplastic cells. J Cell Biol. 1982 Nov;95(2 Pt 1):527–535. doi: 10.1083/jcb.95.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromkowski S. H., Mama K., Yagi J., Sen R., Rath S. Double-stranded RNA and bacterial lipopolysaccharide enhance sensitivity to TNF-alpha-mediated cell death. Int Immunol. 1990;2(9):903–908. doi: 10.1093/intimm/2.9.903. [DOI] [PubMed] [Google Scholar]
- Grooten J., Goossens V., Vanhaesebroeck B., Fiers W. Cell membrane permeabilization and cellular collapse, followed by loss of dehydrogenase activity: early events in tumour necrosis factor-induced cytotoxicity. Cytokine. 1993 Nov;5(6):546–555. doi: 10.1016/s1043-4666(05)80003-1. [DOI] [PubMed] [Google Scholar]
- Gutman M., Hartstein E. Inhibition of mitochondrial malate dehydrogenase by 2-thenoyltrifluoroacetone. FEBS Lett. 1974 Dec 15;49(2):170–173. doi: 10.1016/0014-5793(74)80504-1. [DOI] [PubMed] [Google Scholar]
- Haliday E. M., Ramesha C. S., Ringold G. TNF induces c-fos via a novel pathway requiring conversion of arachidonic acid to a lipoxygenase metabolite. EMBO J. 1991 Jan;10(1):109–115. doi: 10.1002/j.1460-2075.1991.tb07926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatefi Y., Stiggall D. L. Preparation and properties of NADH: cytochrome c oxidoreductase (complex I--III). Methods Enzymol. 1978;53:5–10. doi: 10.1016/s0076-6879(78)53005-x. [DOI] [PubMed] [Google Scholar]
- Hayakawa M., Ishida N., Takeuchi K., Shibamoto S., Hori T., Oku N., Ito F., Tsujimoto M. Arachidonic acid-selective cytosolic phospholipase A2 is crucial in the cytotoxic action of tumor necrosis factor. J Biol Chem. 1993 May 25;268(15):11290–11295. [PubMed] [Google Scholar]
- Hennet T., Richter C., Peterhans E. Tumour necrosis factor-alpha induces superoxide anion generation in mitochondria of L929 cells. Biochem J. 1993 Jan 15;289(Pt 2):587–592. doi: 10.1042/bj2890587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepburn A., Boeynaems J. M., Fiers W., Dumont J. E. Modulation of tumor necrosis factor-alpha cytotoxicity in L929 cells by bacterial toxins, hydrocortisone and inhibitors of arachidonic acid metabolism. Biochem Biophys Res Commun. 1987 Dec 16;149(2):815–822. doi: 10.1016/0006-291x(87)90440-2. [DOI] [PubMed] [Google Scholar]
- Hirschelmann R., Kuhn C., Zarnack S., Funke T., Bekemeier H., Giessler A. J., Phuoc L. T., Boge A. Action of metal chelators on lipoxygenases, cyclooxygenase and on inflammation-induced vasodepression. Biomed Biochim Acta. 1988;47(10-11):S256–S259. [PubMed] [Google Scholar]
- Holtzman M. J., Pentland A., Baenziger N. L., Hansbrough J. R. Heterogeneity of cellular expression of arachidonate 15-lipoxygenase: implications for biological activity. Biochim Biophys Acta. 1989 Jun 8;1003(2):204–208. doi: 10.1016/0005-2760(89)90257-9. [DOI] [PubMed] [Google Scholar]
- Jones O. T., Hancock J. T. Assays of plasma membrane NADPH oxidase. Methods Enzymol. 1994;233:222–229. doi: 10.1016/s0076-6879(94)33025-5. [DOI] [PubMed] [Google Scholar]
- Jones S. A., Hancock J. T., Jones O. T., Neubauer A., Topley N. The expression of NADPH oxidase components in human glomerular mesangial cells: detection of protein and mRNA for p47phox, p67phox, and p22phox. J Am Soc Nephrol. 1995 Jan;5(7):1483–1491. doi: 10.1681/ASN.V571483. [DOI] [PubMed] [Google Scholar]
- Kaur H., Halliwell B. Detection of hydroxyl radicals by aromatic hydroxylation. Methods Enzymol. 1994;233:67–82. doi: 10.1016/s0076-6879(94)33009-3. [DOI] [PubMed] [Google Scholar]
- King M. P., Attardi G. Human cells lacking mtDNA: repopulation with exogenous mitochondria by complementation. Science. 1989 Oct 27;246(4929):500–503. doi: 10.1126/science.2814477. [DOI] [PubMed] [Google Scholar]
- Knauer M. F., Longmuir K. J., Yamamoto R. S., Fitzgerald T. P., Granger G. A. Mechanism of human lymphotoxin and tumor necrosis factor induced destruction of cells in vitro: phospholipase activation and deacylation of specific-membrane phospholipids. J Cell Physiol. 1990 Mar;142(3):469–479. doi: 10.1002/jcp.1041420305. [DOI] [PubMed] [Google Scholar]
- Kyprianou N., Alexander R. B., Isaacs J. T. Activation of programmed cell death by recombinant human tumor necrosis factor plus topoisomerase II-targeted drugs in L929 tumor cells. J Natl Cancer Inst. 1991 Mar 6;83(5):346–350. doi: 10.1093/jnci/83.5.346. [DOI] [PubMed] [Google Scholar]
- Kühn H., Schewe T., Rapoport S. M. The stereochemistry of the reactions of lipoxygenases and their metabolites. Proposed nomenclature of lipoxygenases and related enzymes. Adv Enzymol Relat Areas Mol Biol. 1986;58:273–311. doi: 10.1002/9780470123041.ch7. [DOI] [PubMed] [Google Scholar]
- Lancaster J. R., Jr, Laster S. M., Gooding L. R. Inhibition of target cell mitochondrial electron transfer by tumor necrosis factor. FEBS Lett. 1989 May 8;248(1-2):169–174. doi: 10.1016/0014-5793(89)80454-5. [DOI] [PubMed] [Google Scholar]
- Larrick J. W., Wright S. C. Cytotoxic mechanism of tumor necrosis factor-alpha. FASEB J. 1990 Nov;4(14):3215–3223. doi: 10.1096/fasebj.4.14.2172061. [DOI] [PubMed] [Google Scholar]
- MARGOLIASH E., FROHWIRT N. Spectrum of horse-heart cytochrome c. Biochem J. 1959 Mar;71(3):570–572. doi: 10.1042/bj0710570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M. The entry of enveloped viruses into cells by endocytosis. Biochem J. 1984 Feb 15;218(1):1–10. doi: 10.1042/bj2180001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews N., Neale M. L., Jackson S. K., Stark J. M. Tumour cell killing by tumour necrosis factor: inhibition by anaerobic conditions, free-radical scavengers and inhibitors of arachidonate metabolism. Immunology. 1987 Sep;62(1):153–155. [PMC free article] [PubMed] [Google Scholar]
- Meier B., Cross A. R., Hancock J. T., Kaup F. J., Jones O. T. Identification of a superoxide-generating NADPH oxidase system in human fibroblasts. Biochem J. 1991 Apr 1;275(Pt 1):241–245. doi: 10.1042/bj2750241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier B., Radeke H. H., Selle S., Younes M., Sies H., Resch K., Habermehl G. G. Human fibroblasts release reactive oxygen species in response to interleukin-1 or tumour necrosis factor-alpha. Biochem J. 1989 Oct 15;263(2):539–545. doi: 10.1042/bj2630539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy H. S., Shayman J. A., Till G. O., Mahrougui M., Owens C. B., Ryan U. S., Ward P. A. Superoxide responses of endothelial cells to C5a and TNF-alpha: divergent signal transduction pathways. Am J Physiol. 1992 Jul;263(1 Pt 1):L51–L59. doi: 10.1152/ajplung.1992.263.1.L51. [DOI] [PubMed] [Google Scholar]
- Murrell G. A., Francis M. J., Bromley L. Modulation of fibroblast proliferation by oxygen free radicals. Biochem J. 1990 Feb 1;265(3):659–665. doi: 10.1042/bj2650659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale M. L., Fiera R. A., Matthews N. Involvement of phospholipase A2 activation in tumour cell killing by tumour necrosis factor. Immunology. 1988 May;64(1):81–85. [PMC free article] [PubMed] [Google Scholar]
- Nelson M. J., Seitz S. P., Cowling R. A. Enzyme-bound pentadienyl and peroxyl radicals in purple lipoxygenase. Biochemistry. 1990 Jul 24;29(29):6897–6903. doi: 10.1021/bi00481a020. [DOI] [PubMed] [Google Scholar]
- O'Donnell V. B., Smith G. C., Jones O. T. Involvement of phenyl radicals in iodonium inhibition of flavoenzymes. Mol Pharmacol. 1994 Oct;46(4):778–785. [PubMed] [Google Scholar]
- Pogrebniak H., Matthews W., Mitchell J., Russo A., Samuni A., Pass H. Spin trap protection from tumor necrosis factor cytotoxicity. J Surg Res. 1991 May;50(5):469–474. doi: 10.1016/0022-4804(91)90026-i. [DOI] [PubMed] [Google Scholar]
- Pogrebniak H., Matthews W., Mitchell J., Russo A., Samuni A., Pass H. Spin trap protection from tumor necrosis factor cytotoxicity. J Surg Res. 1991 May;50(5):469–474. doi: 10.1016/0022-4804(91)90026-i. [DOI] [PubMed] [Google Scholar]
- Radeke H. H., Meier B., Topley N., Flöge J., Habermehl G. G., Resch K. Interleukin 1-alpha and tumor necrosis factor-alpha induce oxygen radical production in mesangial cells. Kidney Int. 1990 Feb;37(2):767–775. doi: 10.1038/ki.1990.44. [DOI] [PubMed] [Google Scholar]
- Richter C., Wendel A., Weser U., Azzi A. Inhibition by superoxide dismutase of linoleic acid peroxidation induced by lipoxidase. FEBS Lett. 1975 Mar 1;51(1):300–303. doi: 10.1016/0014-5793(75)80912-4. [DOI] [PubMed] [Google Scholar]
- Roy P., Roy S. K., Mitra A., Kulkarni A. P. Superoxide generation by lipoxygenase in the presence of NADH and NADPH. Biochim Biophys Acta. 1994 Sep 15;1214(2):171–179. doi: 10.1016/0005-2760(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Salari H., Braquet P., Borgeat P. Comparative effects of indomethacin, acetylenic acids, 15-HETE, nordihydroguaiaretic acid and BW755C on the metabolism of arachidonic acid in human leukocytes and platelets. Prostaglandins Leukot Med. 1984 Jan;13(1):53–60. doi: 10.1016/0262-1746(84)90102-1. [DOI] [PubMed] [Google Scholar]
- Sandstrom P. A., Tebbey P. W., Van Cleave S., Buttke T. M. Lipid hydroperoxides induce apoptosis in T cells displaying a HIV-associated glutathione peroxidase deficiency. J Biol Chem. 1994 Jan 14;269(2):798–801. [PubMed] [Google Scholar]
- Schulze-Osthoff K., Bakker A. C., Vanhaesebroeck B., Beyaert R., Jacob W. A., Fiers W. Cytotoxic activity of tumor necrosis factor is mediated by early damage of mitochondrial functions. Evidence for the involvement of mitochondrial radical generation. J Biol Chem. 1992 Mar 15;267(8):5317–5323. [PubMed] [Google Scholar]
- Schulze-Osthoff K., Beyaert R., Vandevoorde V., Haegeman G., Fiers W. Depletion of the mitochondrial electron transport abrogates the cytotoxic and gene-inductive effects of TNF. EMBO J. 1993 Aug;12(8):3095–3104. doi: 10.1002/j.1460-2075.1993.tb05978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schütze S., Berkovic D., Tomsing O., Unger C., Krönke M. Tumor necrosis factor induces rapid production of 1'2'diacylglycerol by a phosphatidylcholine-specific phospholipase C. J Exp Med. 1991 Nov 1;174(5):975–988. doi: 10.1084/jem.174.5.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spolsky C. M., Eisenstadt J. M. Chloramphenicol-resistant mutants of human HeLa cells. FEBS Lett. 1972 Sep 15;25(2):319–324. doi: 10.1016/0014-5793(72)80514-3. [DOI] [PubMed] [Google Scholar]
- Staroń K., Kaniuga Z. Different sensitivity of nuclear and microsomal NADH-cytochrome c reductase activities to thenoyltrifluoroacetone. FEBS Lett. 1974 Sep 1;45(1):1–2. doi: 10.1016/0014-5793(74)80795-7. [DOI] [PubMed] [Google Scholar]
- Suffys P., Beyaert R., Van Roy F., Fiers W. Reduced tumour necrosis factor-induced cytotoxicity by inhibitors of the arachidonic acid metabolism. Biochem Biophys Res Commun. 1987 Dec 16;149(2):735–743. doi: 10.1016/0006-291x(87)90429-3. [DOI] [PubMed] [Google Scholar]
- Tada H., Shiho O., Kuroshima K., Koyama M., Tsukamoto K. An improved colorimetric assay for interleukin 2. J Immunol Methods. 1986 Nov 6;93(2):157–165. doi: 10.1016/0022-1759(86)90183-3. [DOI] [PubMed] [Google Scholar]
- Tang D. G., Honn K. V. 12-Lipoxygenase, 12(S)-HETE, and cancer metastasis. Ann N Y Acad Sci. 1994 Nov 15;744:199–215. doi: 10.1111/j.1749-6632.1994.tb52738.x. [DOI] [PubMed] [Google Scholar]
- Warren S., Torti S. V., Torti F. M. The role of iron in the cytotoxicity of tumor necrosis factor. Lymphokine Cytokine Res. 1993 Apr;12(2):75–80. [PubMed] [Google Scholar]
- Watanabe N., Niitsu Y., Neda H., Sone H., Yamauchi N., Maeda M., Urushizaki I. Cytocidal mechanism of TNF: effects of lysosomal enzyme and hydroxyl radical inhibitors on cytotoxicity. Immunopharmacol Immunotoxicol. 1988;10(1):109–116. doi: 10.3109/08923978809014405. [DOI] [PubMed] [Google Scholar]
- Wong G. H., Elwell J. H., Oberley L. W., Goeddel D. V. Manganous superoxide dismutase is essential for cellular resistance to cytotoxicity of tumor necrosis factor. Cell. 1989 Sep 8;58(5):923–931. doi: 10.1016/0092-8674(89)90944-6. [DOI] [PubMed] [Google Scholar]
- Wong G. H., Goeddel D. V. Induction of manganous superoxide dismutase by tumor necrosis factor: possible protective mechanism. Science. 1988 Nov 11;242(4880):941–944. doi: 10.1126/science.3263703. [DOI] [PubMed] [Google Scholar]
- Yamauchi N., Kuriyama H., Watanabe N., Neda H., Maeda M., Niitsu Y. Intracellular hydroxyl radical production induced by recombinant human tumor necrosis factor and its implication in the killing of tumor cells in vitro. Cancer Res. 1989 Apr 1;49(7):1671–1675. [PubMed] [Google Scholar]