Abstract
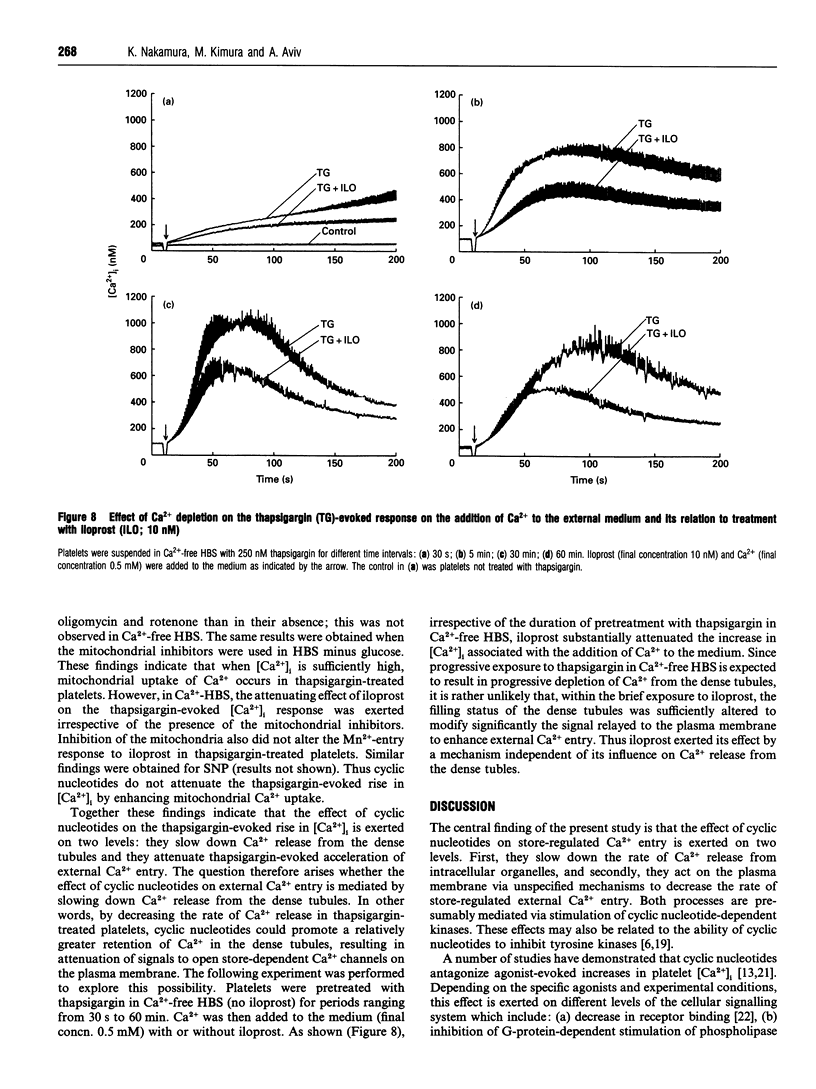
This study explores the role of cyclic nucleotides (i.e. cyclic AMP and cyclic GMP) in store-regulated external Ca2+ entry in human platelets. To stimulate store-regulated external Ca2+ entry, thapsigargin was used to deplete Ca2+ from the dense tubules, and sodium nitroprusside and iloprost respectively were used to stimulate endogenous cyclic GMP and cyclic AMP formation. Pretreatment with sodium nitroprusside and iloprost (a) attenuated the thapsigargin-evoked external Ca2+ entry and (b) reduced the rate of Ca2+ release from the dense tubules. The effects on external Ca2+ entry and Ca2+ release from the dense tubules were exerted independently and were apparently mediated through activation of the respective cyclic nucleotide-dependent protein kinases. Both sodium nitroprusside and iloprost reduced tyrosine kinase phosphorylation of a number of proteins, particularly a 72 kDa protein band. Both agents also attenuated the thapsigargin-evoked tyrosine kinase phosphorylation of the 72 kDa band. Intracellular Ca2+ depletion resulted in a reduction in tyrosine kinase-mediated phosphorylation of a number of protein bands, including the 72 kDa band and the further attenuation of thapsigargin-mediated tyrosine phosphorylation of this band. The effects of the cyclic nucleotides on cellular Ca2+ homoeostasis in thapsigargin-treated platelets were not exerted via acceleration of Ca2+ extrusion or Ca2+ sequestration into the mitochondria. We conclude that cyclic nucleotides participate in store-regulated control of external Ca2+ entry by slowing down the rate of external Ca2+ entry and Ca2+ release from intracellular Ca2+ stores. These effects are apparently mediated via cyclic nucleotide-dependent protein kinases and the attenuation of protein phosphorylation by tyrosine kinases.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso M. T., Alvarez J., Montero M., Sanchez A., García-Sancho J. Agonist-induced Ca2+ influx into human platelets is secondary to the emptying of intracellular Ca2+ stores. Biochem J. 1991 Dec 15;280(Pt 3):783–789. doi: 10.1042/bj2800783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Authi K. S., Bokkala S., Patel Y., Kakkar V. V., Munkonge F. Ca2+ release from platelet intracellular stores by thapsigargin and 2,5-di-(t-butyl)-1,4-benzohydroquinone: relationship to Ca2+ pools and relevance in platelet activation. Biochem J. 1993 Aug 15;294(Pt 1):119–126. doi: 10.1042/bj2940119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachelot C., Cano E., Grelac F., Saleun S., Druker B. J., Levy-Toledano S., Fischer S., Rendu F. Functional implications of tyrosine protein phosphorylation in platelets. Simultaneous studies with different agonists and inhibitors. Biochem J. 1992 Jun 15;284(Pt 3):923–928. doi: 10.1042/bj2840923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Brüne B., Ullrich V. Cyclic nucleotides and intracellular-calcium homeostasis in human platelets. Eur J Biochem. 1992 Jul 15;207(2):607–613. doi: 10.1111/j.1432-1033.1992.tb17087.x. [DOI] [PubMed] [Google Scholar]
- Dhar A., Shukla S. D. Tyrosine kinases in platelet signalling. Br J Haematol. 1993 May;84(1):1–7. doi: 10.1111/j.1365-2141.1993.tb03018.x. [DOI] [PubMed] [Google Scholar]
- Doni M. G., Deana R., Padoin E., Ruzzene M., Alexandre A. Platelet activation by diacylglycerol or ionomycin is inhibited by nitroprusside. Biochim Biophys Acta. 1991 Sep 24;1094(3):323–329. doi: 10.1016/0167-4889(91)90093-d. [DOI] [PubMed] [Google Scholar]
- Geiger J., Nolte C., Butt E., Sage S. O., Walter U. Role of cGMP and cGMP-dependent protein kinase in nitrovasodilator inhibition of agonist-evoked calcium elevation in human platelets. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):1031–1035. doi: 10.1073/pnas.89.3.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger J., Nolte C., Walter U. Regulation of calcium mobilization and entry in human platelets by endothelium-derived factors. Am J Physiol. 1994 Jul;267(1 Pt 1):C236–C244. doi: 10.1152/ajpcell.1994.267.1.C236. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Jenner S., Farndale R. W., Sage S. O. The effect of calcium-store depletion and refilling with various bivalent cations on tyrosine phosphorylation and Mn2+ entry in fura-2-loaded human platelets. Biochem J. 1994 Oct 15;303(Pt 2):337–339. doi: 10.1042/bj3030337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson J. S., Haynes D. H. Cyclic GMP increases the rate of the calcium extrusion pump in intact human platelets but has no direct effect on the dense tubular calcium accumulation system. Biochim Biophys Acta. 1992 Mar 23;1105(1):40–50. doi: 10.1016/0005-2736(92)90160-n. [DOI] [PubMed] [Google Scholar]
- Johansson J. S., Nied L. E., Haynes D. H. Cyclic AMP stimulates Ca(2+)-ATPase-mediated Ca2+ extrusion from human platelets. Biochim Biophys Acta. 1992 Mar 23;1105(1):19–28. doi: 10.1016/0005-2736(92)90158-i. [DOI] [PubMed] [Google Scholar]
- Kimura M., Lasker N., Aviv A. Cyclic nucleotides attenuate thrombin-evoked alterations in parameters of platelet Na/H antiport. The role of cytosolic Ca. J Clin Invest. 1992 Apr;89(4):1121–1127. doi: 10.1172/JCI115692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M., Nakamura K., Fenton J. W., 2nd, Andersen T. T., Reeves J. P., Aviv A. Role of external Na+ and cytosolic pH in agonist-evoked cytosolic Ca2+ response in human platelets. Am J Physiol. 1994 Dec;267(6 Pt 1):C1543–C1552. doi: 10.1152/ajpcell.1994.267.6.C1543. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarowski E. R., Lapetina E. G. Activation of platelet phospholipase C by fluoride is inhibited by elevation of cyclic AMP. Biochem Biophys Res Commun. 1989 Jan 31;158(2):440–444. doi: 10.1016/s0006-291x(89)80067-1. [DOI] [PubMed] [Google Scholar]
- Lee K. M., Toscas K., Villereal M. L. Inhibition of bradykinin- and thapsigargin-induced Ca2+ entry by tyrosine kinase inhibitors. J Biol Chem. 1993 May 15;268(14):9945–9948. [PubMed] [Google Scholar]
- Lerea K. M., Glomset J. A., Krebs E. G. Agents that elevate cAMP levels in platelets decrease thrombin binding. J Biol Chem. 1987 Jan 5;262(1):282–288. [PubMed] [Google Scholar]
- Morgan R. O., Newby A. C. Nitroprusside differentially inhibits ADP-stimulated calcium influx and mobilization in human platelets. Biochem J. 1989 Mar 1;258(2):447–454. doi: 10.1042/bj2580447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda A., Druker B. J., Smith M., Salzman E. W. Inhibition by sodium nitroprusside or PGE1 of tyrosine phosphorylation induced in platelets by thrombin or ADP. Am J Physiol. 1992 Mar;262(3 Pt 1):C701–C707. doi: 10.1152/ajpcell.1992.262.3.C701. [DOI] [PubMed] [Google Scholar]
- Parekh A. B., Terlau H., Stühmer W. Depletion of InsP3 stores activates a Ca2+ and K+ current by means of a phosphatase and a diffusible messenger. Nature. 1993 Aug 26;364(6440):814–818. doi: 10.1038/364814a0. [DOI] [PubMed] [Google Scholar]
- Pumiglia K. M., Huang C. K., Feinstein M. B. Elevation of cAMP, but not cGMP, inhibits thrombin-stimulated tyrosine phosphorylation in human platelets. Biochem Biophys Res Commun. 1990 Sep 14;171(2):738–745. doi: 10.1016/0006-291x(90)91208-a. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Capacitative calcium entry revisited. Cell Calcium. 1990 Nov-Dec;11(10):611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- Randriamampita C., Tsien R. Y. Emptying of intracellular Ca2+ stores releases a novel small messenger that stimulates Ca2+ influx. Nature. 1993 Aug 26;364(6440):809–814. doi: 10.1038/364809a0. [DOI] [PubMed] [Google Scholar]
- Sage S. O., Reast R., Rink T. J. ADP evokes biphasic Ca2+ influx in fura-2-loaded human platelets. Evidence for Ca2+ entry regulated by the intracellular Ca2+ store. Biochem J. 1990 Feb 1;265(3):675–680. doi: 10.1042/bj2650675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargeant P., Clarkson W. D., Sage S. O., Heemskerk J. W. Calcium influx evoked by Ca2+ store depletion in human platelets is more susceptible to cytochrome P-450 inhibitors than receptor-mediated calcium entry. Cell Calcium. 1992 Oct;13(9):553–564. doi: 10.1016/0143-4160(92)90035-q. [DOI] [PubMed] [Google Scholar]
- Sargeant P., Farndale R. W., Sage S. O. ADP- and thapsigargin-evoked Ca2+ entry and protein-tyrosine phosphorylation are inhibited by the tyrosine kinase inhibitors genistein and methyl-2,5-dihydroxycinnamate in fura-2-loaded human platelets. J Biol Chem. 1993 Aug 25;268(24):18151–18156. [PubMed] [Google Scholar]
- Sargeant P., Farndale R. W., Sage S. O. The tyrosine kinase inhibitors methyl 2,5-dihydroxycinnamate and genistein reduce thrombin-evoked tyrosine phosphorylation and Ca2+ entry in human platelets. FEBS Lett. 1993 Jan 11;315(3):242–246. doi: 10.1016/0014-5793(93)81172-v. [DOI] [PubMed] [Google Scholar]
- Vostal J. G., Jackson W. L., Shulman N. R. Cytosolic and stored calcium antagonistically control tyrosine phosphorylation of specific platelet proteins. J Biol Chem. 1991 Sep 5;266(25):16911–16916. [PubMed] [Google Scholar]