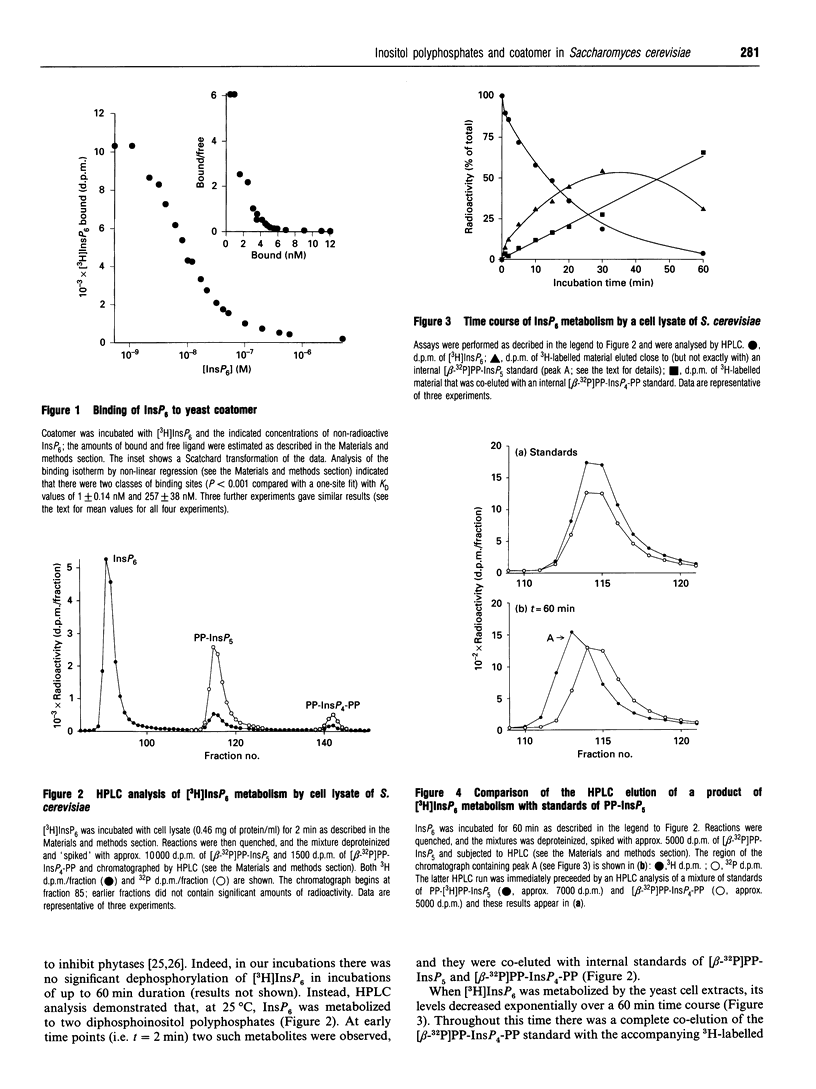
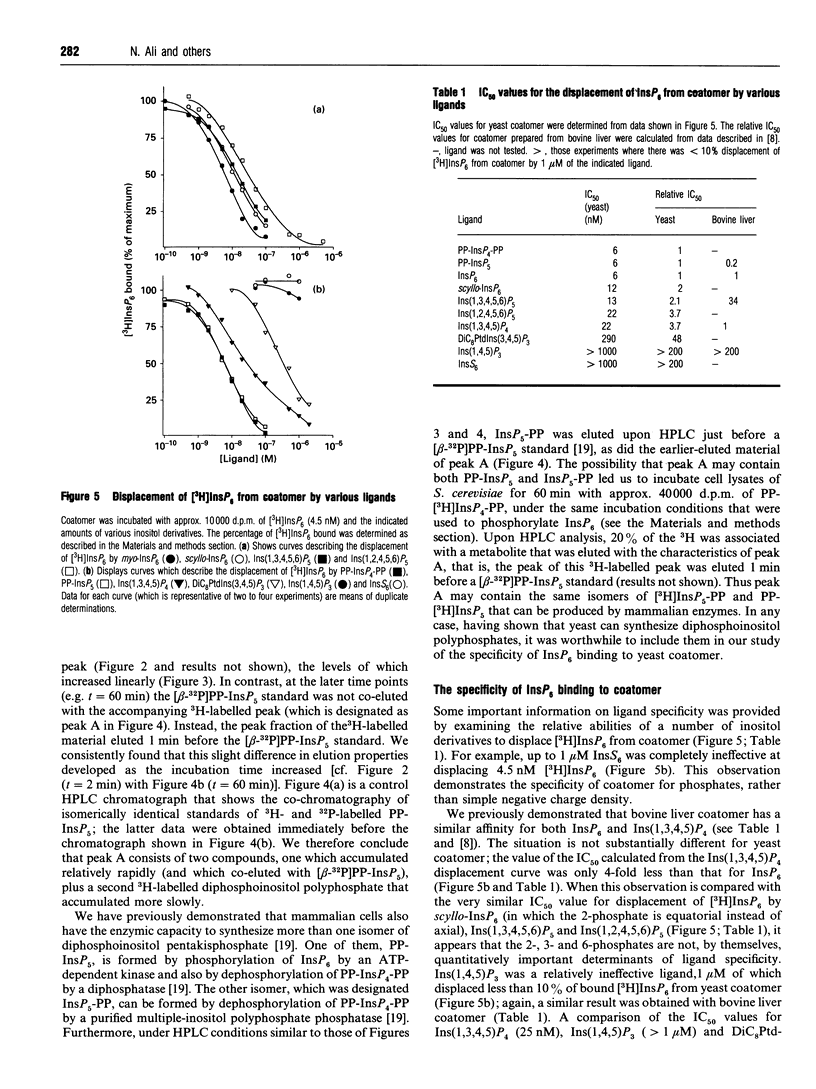
Abstract
Coatomer is an oligomeric complex of coat proteins that regulates vesicular traffic through the Golgi complex and from the Golgi to the endoplasmic reticulum [Pelham (1994) Cell 79, 1125-1127]. We have investigated whether the binding of InsP6 to mammalian coatomer [Fleischer, Xie, Mayrleitner, Shears and Fleischer (1994) J. Biol. Chem. 269, 17826-17832] is conserved in the genetically amenable model Saccharomyces cerevisiae. We have isolated coatomer from S. cerevisiae and found it to bind InsP6 at two apparent classes of binding sites (KD1 = 0.8 +/- 0.2 nM; KD2 = 361 +/- 102 nM). Ligand specificity was studied by displacing 4.5 nM [3H]InsP6 from coatomer with various Ins derivatives. The following IC50 values (nM) were obtained: myo-InsP6 = 6; bis(diphospho)inositol tetrakisphosphate = 6; diphosphoinositol pentakisphosphate = 6; scyllo-InsP6 = 12; Ins(1,3,4,5,6)P5 = 13; Ins(1,2,4,5,6)P5 = 22; Ins(1,3,4,5)P4 = 22; 1-O-(1,2-di-O-octanoyl-sn-glycero-3-phospho)-D-Ins(3,4,5)P3 = 290. Less than 10% of the 3H label was displaced by 1 microM of either Ins(1,4,5)P3 or inositol hexakis-sulphate. A cell-free lysate of S. cerevisiae synthesized diphosphoinositol polyphosphates (PP-InsPn) from InsP6, but our binding data, plus measurements of the relative levels of inositol polyphosphates in intact yeast [Hawkins, Stephens and Piggott (1993) J. Biol. Chem. 268, 3374-3383], indicate that InsP6 is the major physiologically relevant ligand. Thus a reconstituted vesicle trafficking system using coatomer and other functionally related components isolated from yeast should be a useful model for elucidating the functional significance of the binding of InsP6 by coatomer.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlowe C., Orci L., Yeung T., Hosobuchi M., Hamamoto S., Salama N., Rexach M. F., Ravazzola M., Amherdt M., Schekman R. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994 Jun 17;77(6):895–907. doi: 10.1016/0092-8674(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Beck K. A., Keen J. H. Interaction of phosphoinositide cycle intermediates with the plasma membrane-associated clathrin assembly protein AP-2. J Biol Chem. 1991 Mar 5;266(7):4442–4447. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Duden R., Hosobuchi M., Hamamoto S., Winey M., Byers B., Schekman R. Yeast beta- and beta'-coat proteins (COP). Two coatomer subunits essential for endoplasmic reticulum-to-Golgi protein traffic. J Biol Chem. 1994 Sep 30;269(39):24486–24495. [PubMed] [Google Scholar]
- Fleischer B., Xie J., Mayrleitner M., Shears S. B., Palmer D. J., Fleischer S. Golgi coatomer binds, and forms K(+)-selective channels gated by, inositol polyphosphates. J Biol Chem. 1994 Jul 8;269(27):17826–17832. [PubMed] [Google Scholar]
- Gibson D. M., Ullah A. H. Purification and characterization of phytase from cotyledons of germinating soybean seeds. Arch Biochem Biophys. 1988 Feb 1;260(2):503–513. doi: 10.1016/0003-9861(88)90475-4. [DOI] [PubMed] [Google Scholar]
- Glennon M. C., Shears S. B. Turnover of inositol pentakisphosphates, inositol hexakisphosphate and diphosphoinositol polyphosphates in primary cultured hepatocytes. Biochem J. 1993 Jul 15;293(Pt 2):583–590. doi: 10.1042/bj2930583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins P. T., Stephens L. R., Piggott J. R. Analysis of inositol metabolites produced by Saccharomyces cerevisiae in response to glucose stimulation. J Biol Chem. 1993 Feb 15;268(5):3374–3383. [PubMed] [Google Scholar]
- Hay J. C., Fisette P. L., Jenkins G. H., Fukami K., Takenawa T., Anderson R. A., Martin T. F. ATP-dependent inositide phosphorylation required for Ca(2+)-activated secretion. Nature. 1995 Mar 9;374(6518):173–177. doi: 10.1038/374173a0. [DOI] [PubMed] [Google Scholar]
- Hosobuchi M., Kreis T., Schekman R. SEC21 is a gene required for ER to Golgi protein transport that encodes a subunit of a yeast coatomer. Nature. 1992 Dec 10;360(6404):603–605. doi: 10.1038/360603a0. [DOI] [PubMed] [Google Scholar]
- Kapeller R., Cantley L. C. Phosphatidylinositol 3-kinase. Bioessays. 1994 Aug;16(8):565–576. doi: 10.1002/bies.950160810. [DOI] [PubMed] [Google Scholar]
- Letourneur F., Gaynor E. C., Hennecke S., Démollière C., Duden R., Emr S. D., Riezman H., Cosson P. Coatomer is essential for retrieval of dilysine-tagged proteins to the endoplasmic reticulum. Cell. 1994 Dec 30;79(7):1199–1207. doi: 10.1016/0092-8674(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Menniti F. S., Miller R. N., Putney J. W., Jr, Shears S. B. Turnover of inositol polyphosphate pyrophosphates in pancreatoma cells. J Biol Chem. 1993 Feb 25;268(6):3850–3856. [PubMed] [Google Scholar]
- Niinobe M., Yamaguchi Y., Fukuda M., Mikoshiba K. Synaptotagmin is an inositol polyphosphate binding protein: isolation and characterization as an Ins 1,3,4,5-P4 binding protein. Biochem Biophys Res Commun. 1994 Dec 15;205(2):1036–1042. doi: 10.1006/bbrc.1994.2770. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. About turn for the COPs? Cell. 1994 Dec 30;79(7):1125–1127. doi: 10.1016/0092-8674(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Poyner D. R., Cooke F., Hanley M. R., Reynolds D. J., Hawkins P. T. Characterization of metal ion-induced [3H]inositol hexakisphosphate binding to rat cerebellar membranes. J Biol Chem. 1993 Jan 15;268(2):1032–1038. [PubMed] [Google Scholar]
- Shears S. B. Metabolism of inositol phosphates. Adv Second Messenger Phosphoprotein Res. 1992;26:63–92. [PubMed] [Google Scholar]
- Stephens L., Radenberg T., Thiel U., Vogel G., Khoo K. H., Dell A., Jackson T. R., Hawkins P. T., Mayr G. W. The detection, purification, structural characterization, and metabolism of diphosphoinositol pentakisphosphate(s) and bisdiphosphoinositol tetrakisphosphate(s). J Biol Chem. 1993 Feb 25;268(6):4009–4015. [PubMed] [Google Scholar]
- Toker A., Meyer M., Reddy K. K., Falck J. R., Aneja R., Aneja S., Parra A., Burns D. J., Ballas L. M., Cantley L. C. Activation of protein kinase C family members by the novel polyphosphoinositides PtdIns-3,4-P2 and PtdIns-3,4,5-P3. J Biol Chem. 1994 Dec 23;269(51):32358–32367. [PubMed] [Google Scholar]
- Waters M. G., Serafini T., Rothman J. E. 'Coatomer': a cytosolic protein complex containing subunits of non-clathrin-coated Golgi transport vesicles. Nature. 1991 Jan 17;349(6306):248–251. doi: 10.1038/349248a0. [DOI] [PubMed] [Google Scholar]
- Wuestehube L. J., Schekman R. W. Reconstitution of transport from endoplasmic reticulum to Golgi complex using endoplasmic reticulum-enriched membrane fraction from yeast. Methods Enzymol. 1992;219:124–136. doi: 10.1016/0076-6879(92)19015-x. [DOI] [PubMed] [Google Scholar]
- Ye W., Ali N., Bembenek M. E., Shears S. B., Lafer E. M. Inhibition of clathrin assembly by high affinity binding of specific inositol polyphosphates to the synapse-specific clathrin assembly protein AP-3. J Biol Chem. 1995 Jan 27;270(4):1564–1568. [PubMed] [Google Scholar]


