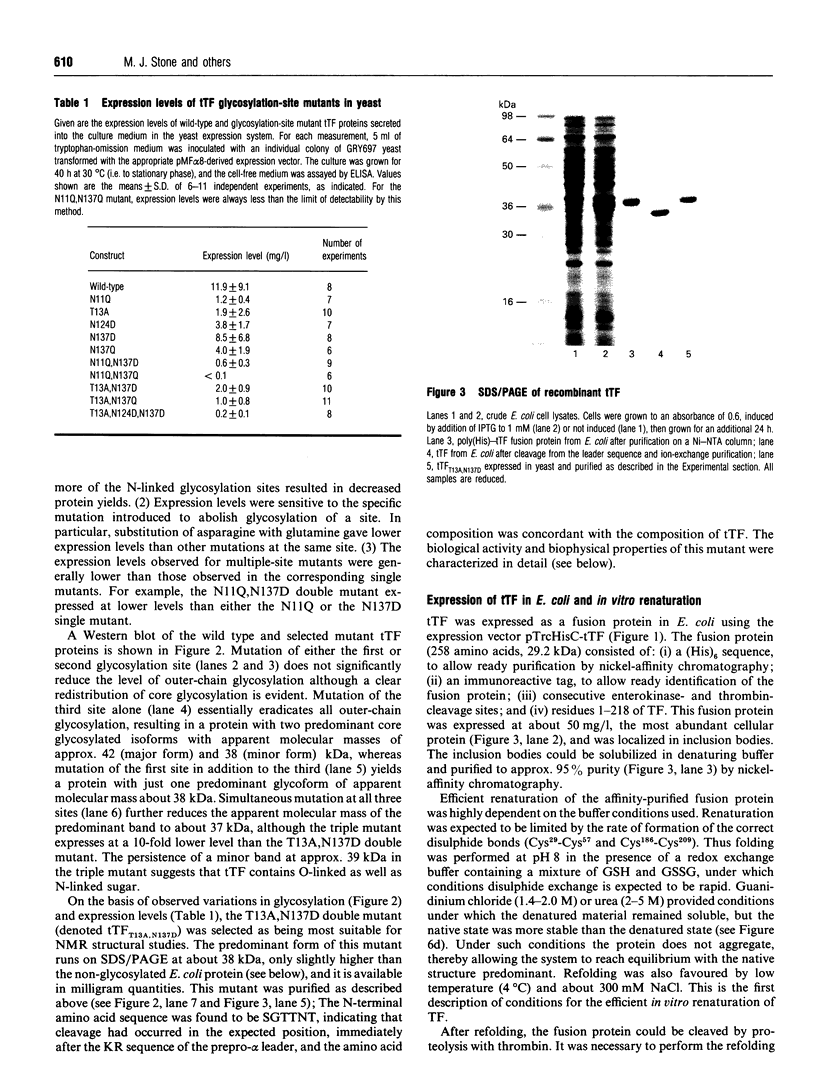
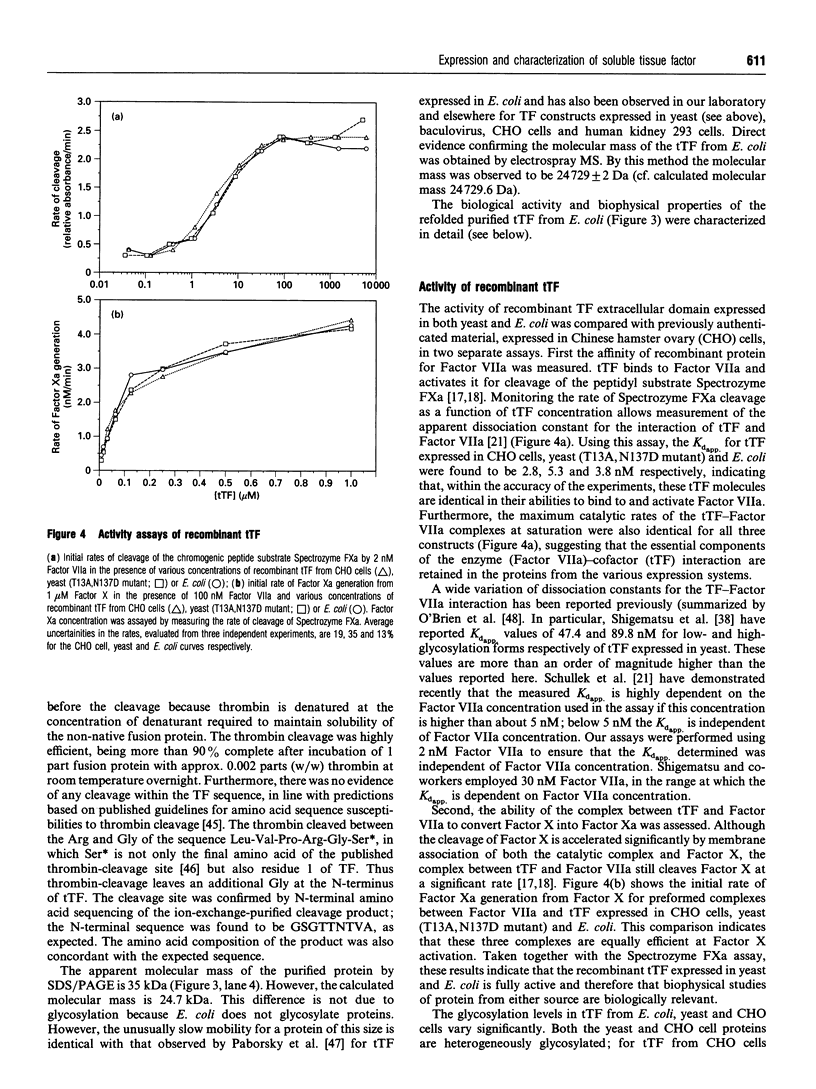
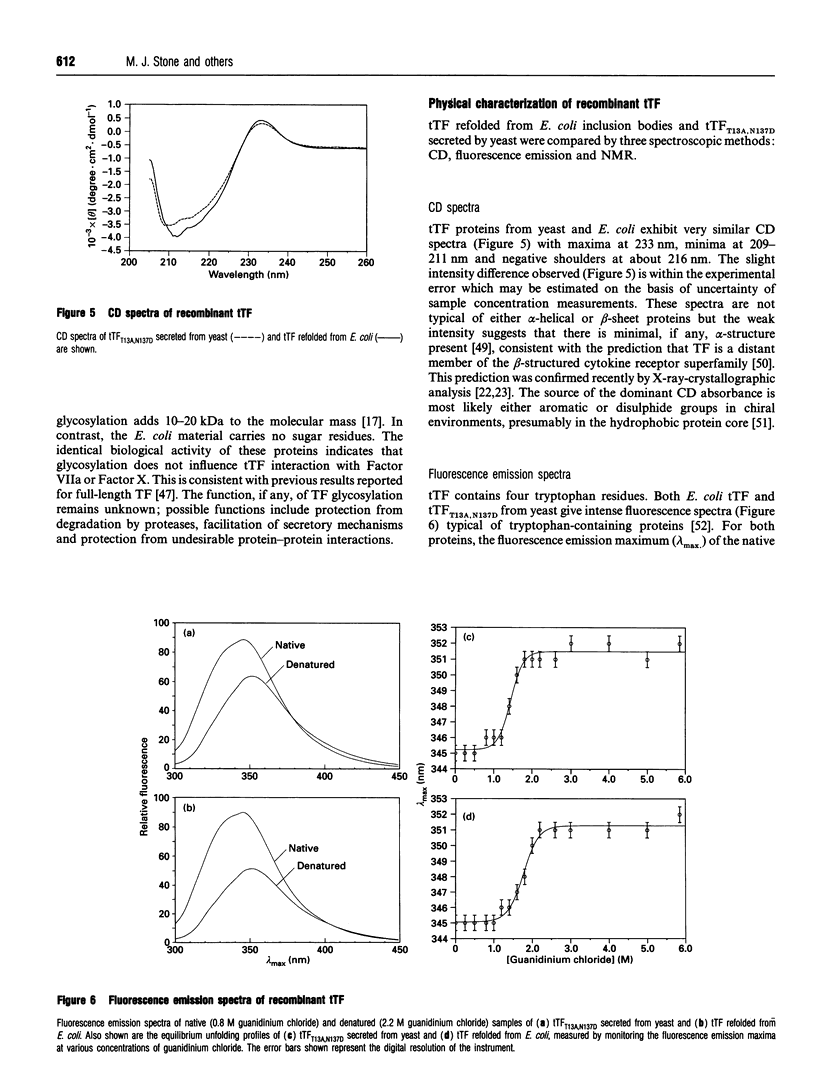
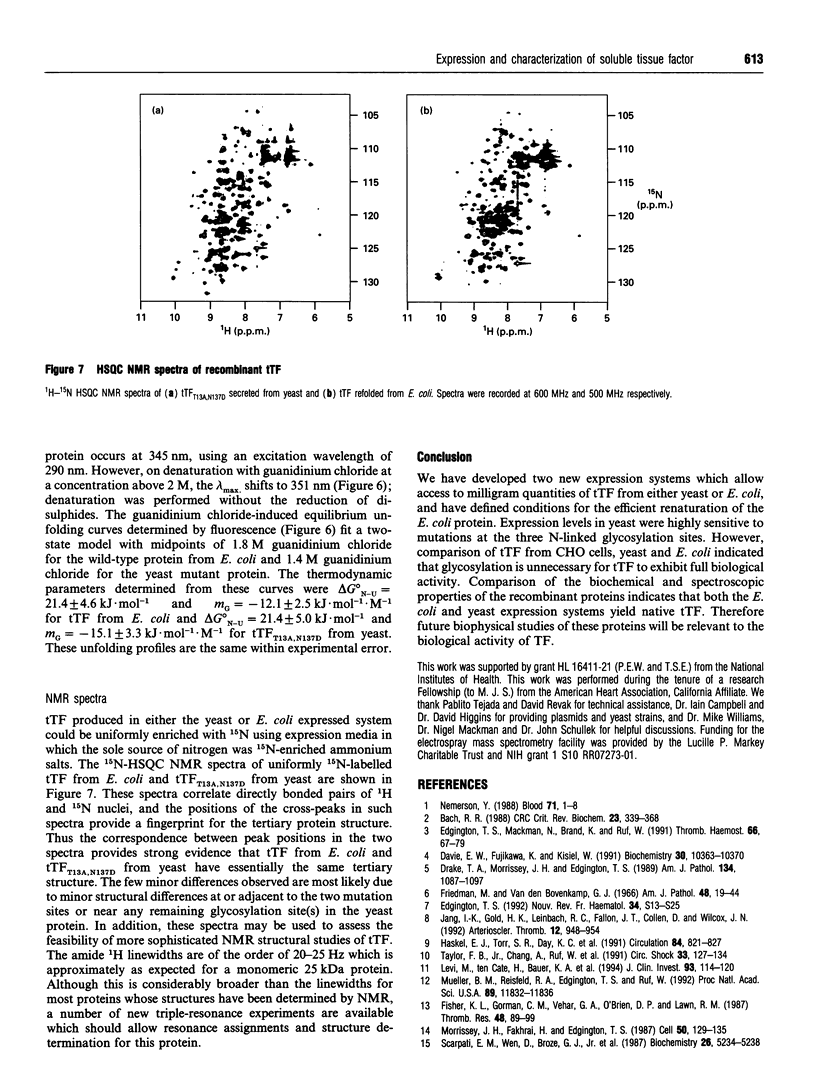
Abstract
Tissue factor (TF) is the cell-surface transmembrane receptor that initiates both the extrinsic and intrinsic blood coagulation cascades. The abilities of TF to associate with Factor VIIa and Factor X in a ternary complex and to enable proteolytic activation of Factor X by Factor VIIa reside in the extracellular domain of TF. We describe the expression of the surface domain of TF (truncated TF, tTF) in both Saccharomyces cerevisiae and Escherichia coli and the biochemical and physical characterization of the recombinant proteins. Wild-type tTF and several glycosylation-site mutants were secreted efficiently by S. cerevisiae under the control of the yeast prepro-alpha-signal sequence; the T13A,N137D double mutant was the most homogeneous variant expressed in milligram quantities. Wild-type tTF was expressed in a non-native state in E. coli inclusion bodies as a fusion protein with a poly(His) leader. The fusion protein could be fully renatured and the leader removed by proteolysis with thrombin; the correct molecular mass (24,729 Da) of the purified protein was confirmed by electrospray mass spectrometry. Recombinant tTFs from yeast, E. coli and Chinese hamster ovary cells were identical in their abilities to bind Factor VIIa, to enhance the catalytic activity of Factor VIIa and to enhance the proteolytic activation of Factor X by Factor VIIa. Furthermore, CD, fluorescence emission and NMR spectra of the yeast and E. coli proteins indicated that these proteins are essentially identical structurally.
Full text
PDF



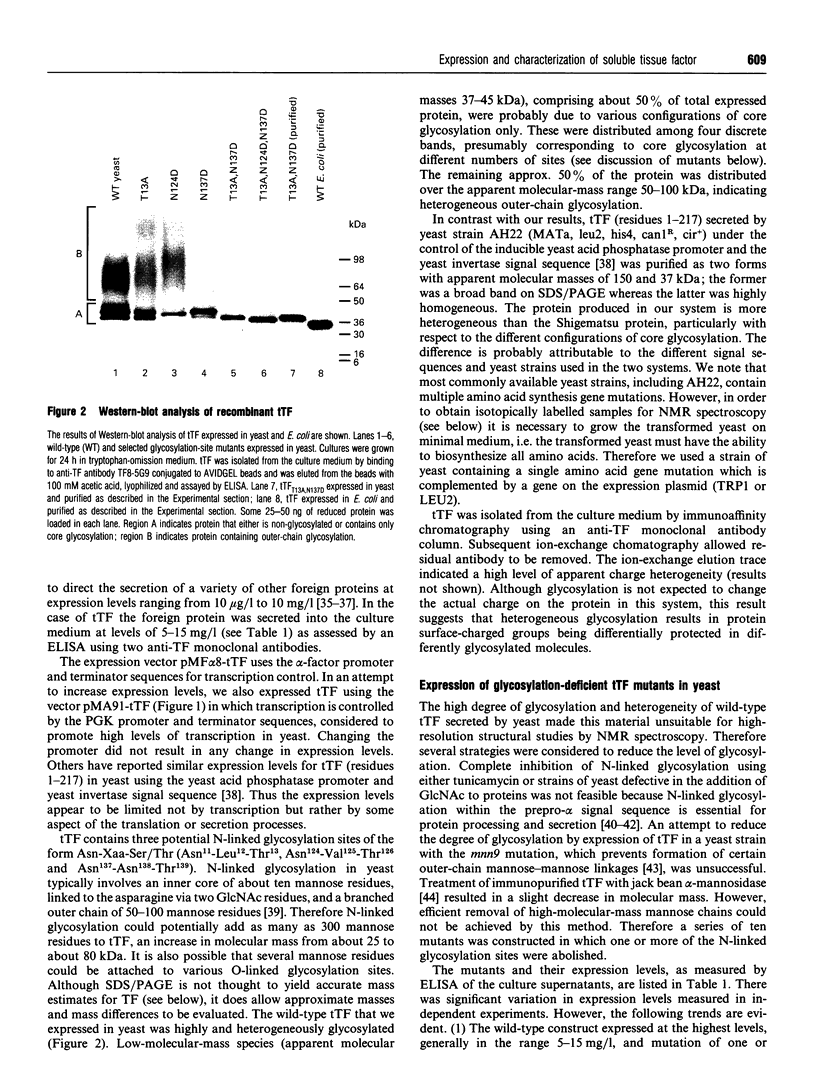

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler A. J., Greenfield N. J., Fasman G. D. Circular dichroism and optical rotatory dispersion of proteins and polypeptides. Methods Enzymol. 1973;27:675–735. doi: 10.1016/s0076-6879(73)27030-1. [DOI] [PubMed] [Google Scholar]
- Bach R. R. Initiation of coagulation by tissue factor. CRC Crit Rev Biochem. 1988;23(4):339–368. doi: 10.3109/10409238809082548. [DOI] [PubMed] [Google Scholar]
- Bazan J. F. Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6934–6938. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergh M. L., Cepko C. L., Wolf D., Robbins P. W. Expression of the Saccharomyces cerevisiae glycoprotein invertase in mouse fibroblasts: glycosylation, secretion, and enzymatic activity. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3570–3574. doi: 10.1073/pnas.84.11.3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitter G. A., Chen K. K., Banks A. R., Lai P. H. Secretion of foreign proteins from Saccharomyces cerevisiae directed by alpha-factor gene fusions. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5330–5334. doi: 10.1073/pnas.81.17.5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brake A. J., Merryweather J. P., Coit D. G., Heberlein U. A., Masiarz F. R., Mullenbach G. T., Urdea M. S., Valenzuela P., Barr P. J. Alpha-factor-directed synthesis and secretion of mature foreign proteins in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4642–4646. doi: 10.1073/pnas.81.15.4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J. Y. Thrombin specificity. Requirement for apolar amino acids adjacent to the thrombin cleavage site of polypeptide substrate. Eur J Biochem. 1985 Sep 2;151(2):217–224. doi: 10.1111/j.1432-1033.1985.tb09091.x. [DOI] [PubMed] [Google Scholar]
- Davie E. W., Fujikawa K., Kisiel W. The coagulation cascade: initiation, maintenance, and regulation. Biochemistry. 1991 Oct 29;30(43):10363–10370. doi: 10.1021/bi00107a001. [DOI] [PubMed] [Google Scholar]
- Drake T. A., Morrissey J. H., Edgington T. S. Selective cellular expression of tissue factor in human tissues. Implications for disorders of hemostasis and thrombosis. Am J Pathol. 1989 May;134(5):1087–1097. [PMC free article] [PubMed] [Google Scholar]
- Edgington T. S., Mackman N., Brand K., Ruf W. The structural biology of expression and function of tissue factor. Thromb Haemost. 1991 Jul 12;66(1):67–79. [PubMed] [Google Scholar]
- Emr S. D., Schekman R., Flessel M. C., Thorner J. An MF alpha 1-SUC2 (alpha-factor-invertase) gene fusion for study of protein localization and gene expression in yeast. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7080–7084. doi: 10.1073/pnas.80.23.7080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher K. L., Gorman C. M., Vehar G. A., O'Brien D. P., Lawn R. M. Cloning and expression of human tissue factor cDNA. Thromb Res. 1987 Oct 1;48(1):89–99. doi: 10.1016/0049-3848(87)90349-5. [DOI] [PubMed] [Google Scholar]
- Friedman M., Van den Bovenkamp G. J. The pathogenesis of a coronary thrombus. Am J Pathol. 1966 Jan;48(1):19–44. [PMC free article] [PubMed] [Google Scholar]
- Harlos K., Martin D. M., O'Brien D. P., Jones E. Y., Stuart D. I., Polikarpov I., Miller A., Tuddenham E. G., Boys C. W. Crystal structure of the extracellular region of human tissue factor. Nature. 1994 Aug 25;370(6491):662–666. doi: 10.1038/370662a0. [DOI] [PubMed] [Google Scholar]
- Haskel E. J., Torr S. R., Day K. C., Palmier M. O., Wun T. C., Sobel B. E., Abendschein D. R. Prevention of arterial reocclusion after thrombolysis with recombinant lipoprotein-associated coagulation inhibitor. Circulation. 1991 Aug;84(2):821–827. doi: 10.1161/01.cir.84.2.821. [DOI] [PubMed] [Google Scholar]
- Hibbs A. R., Meyer D. I. Secretion in yeast: in vitro analysis of the sec53 mutant. EMBO J. 1988 Jul;7(7):2229–2232. doi: 10.1002/j.1460-2075.1988.tb03062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang I. K., Gold H. K., Leinbach R. C., Fallon J. T., Collen D., Wilcox J. N. Antithrombotic effect of a monoclonal antibody against tissue factor in a rabbit model of platelet-mediated arterial thrombosis. Arterioscler Thromb. 1992 Aug;12(8):948–954. doi: 10.1161/01.atv.12.8.948. [DOI] [PubMed] [Google Scholar]
- Jones D. H., Howard B. H. A rapid method for recombination and site-specific mutagenesis by placing homologous ends on DNA using polymerase chain reaction. Biotechniques. 1991 Jan;10(1):62–66. [PubMed] [Google Scholar]
- Koide S., Dyson H. J., Wright P. E. Characterization of a folding intermediate of apoplastocyanin trapped by proline isomerization. Biochemistry. 1993 Nov 23;32(46):12299–12310. doi: 10.1021/bi00097a005. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levi M., ten Cate H., Bauer K. A., van der Poll T., Edgington T. S., Büller H. R., van Deventer S. J., Hack C. E., ten Cate J. W., Rosenberg R. D. Inhibition of endotoxin-induced activation of coagulation and fibrinolysis by pentoxifylline or by a monoclonal anti-tissue factor antibody in chimpanzees. J Clin Invest. 1994 Jan;93(1):114–120. doi: 10.1172/JCI116934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion D., Wüthrich K. Application of phase sensitive two-dimensional correlated spectroscopy (COSY) for measurements of 1H-1H spin-spin coupling constants in proteins. Biochem Biophys Res Commun. 1983 Jun 29;113(3):967–974. doi: 10.1016/0006-291x(83)91093-8. [DOI] [PubMed] [Google Scholar]
- Mellor J., Dobson M. J., Roberts N. A., Tuite M. F., Emtage J. S., White S., Lowe P. A., Patel T., Kingsman A. J., Kingsman S. M. Efficient synthesis of enzymatically active calf chymosin in Saccharomyces cerevisiae. Gene. 1983 Sep;24(1):1–14. doi: 10.1016/0378-1119(83)90126-9. [DOI] [PubMed] [Google Scholar]
- Miyajima A., Bond M. W., Otsu K., Arai K., Arai N. Secretion of mature mouse interleukin-2 by Saccharomyces cerevisiae: use of a general secretion vector containing promoter and leader sequences of the mating pheromone alpha-factor. Gene. 1985;37(1-3):155–161. doi: 10.1016/0378-1119(85)90268-9. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H., Fakhrai H., Edgington T. S. Molecular cloning of the cDNA for tissue factor, the cellular receptor for the initiation of the coagulation protease cascade. Cell. 1987 Jul 3;50(1):129–135. doi: 10.1016/0092-8674(87)90669-6. [DOI] [PubMed] [Google Scholar]
- Mueller B. M., Reisfeld R. A., Edgington T. S., Ruf W. Expression of tissue factor by melanoma cells promotes efficient hematogenous metastasis. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11832–11836. doi: 10.1073/pnas.89.24.11832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller Y. A., Ultsch M. H., Kelley R. F., de Vos A. M. Structure of the extracellular domain of human tissue factor: location of the factor VIIa binding site. Biochemistry. 1994 Sep 13;33(36):10864–10870. doi: 10.1021/bi00202a003. [DOI] [PubMed] [Google Scholar]
- Nemerson Y. Tissue factor and hemostasis. Blood. 1988 Jan;71(1):1–8. [PubMed] [Google Scholar]
- O'Brien D. P., Kemball-Cook G., Hutchinson A. M., Martin D. M., Johnson D. J., Byfield P. G., Takamiya O., Tuddenham E. G., McVey J. H. Surface plasmon resonance studies of the interaction between factor VII and tissue factor. Demonstration of defective tissue factor binding in a variant FVII molecule (FVII-R79Q). Biochemistry. 1994 Nov 29;33(47):14162–14169. doi: 10.1021/bi00251a027. [DOI] [PubMed] [Google Scholar]
- Olins P. O., Devine C. S., Rangwala S. H., Kavka K. S. The T7 phage gene 10 leader RNA, a ribosome-binding site that dramatically enhances the expression of foreign genes in Escherichia coli. Gene. 1988 Dec 15;73(1):227–235. doi: 10.1016/0378-1119(88)90329-0. [DOI] [PubMed] [Google Scholar]
- Paborsky L. R., Tate K. M., Harris R. J., Yansura D. G., Band L., McCray G., Gorman C. M., O'Brien D. P., Chang J. Y., Swartz J. R. Purification of recombinant human tissue factor. Biochemistry. 1989 Oct 3;28(20):8072–8077. doi: 10.1021/bi00446a016. [DOI] [PubMed] [Google Scholar]
- Ruf W., Bender A., Lane D. A., Preissner K. T., Selmayr E., Müller-Berghaus G. Thrombin-induced fibrinopeptide B release from normal and variant fibrinogens: influence of inhibitors of fibrin polymerization. Biochim Biophys Acta. 1988 May 12;965(2-3):169–175. doi: 10.1016/0304-4165(88)90053-0. [DOI] [PubMed] [Google Scholar]
- Ruf W., Kalnik M. W., Lund-Hansen T., Edgington T. S. Characterization of factor VII association with tissue factor in solution. High and low affinity calcium binding sites in factor VII contribute to functionally distinct interactions. J Biol Chem. 1991 Aug 25;266(24):15719–15725. [PubMed] [Google Scholar]
- Ruf W., Miles D. J., Rehemtulla A., Edgington T. S. Mutational analysis of receptor and cofactor function of tissue factor. Methods Enzymol. 1993;222:209–224. doi: 10.1016/0076-6879(93)22015-8. [DOI] [PubMed] [Google Scholar]
- Ruf W., Rehemtulla A., Morrissey J. H., Edgington T. S. Phospholipid-independent and -dependent interactions required for tissue factor receptor and cofactor function. J Biol Chem. 1991 Feb 5;266(4):2158–2166. [PubMed] [Google Scholar]
- Ruf W., Schullek J. R., Stone M. J., Edgington T. S. Mutational mapping of functional residues in tissue factor: identification of factor VII recognition determinants in both structural modules of the predicted cytokine receptor homology domain. Biochemistry. 1994 Feb 15;33(6):1565–1572. doi: 10.1021/bi00172a037. [DOI] [PubMed] [Google Scholar]
- Scarpati E. M., Wen D., Broze G. J., Jr, Miletich J. P., Flandermeyer R. R., Siegel N. R., Sadler J. E. Human tissue factor: cDNA sequence and chromosome localization of the gene. Biochemistry. 1987 Aug 25;26(17):5234–5238. doi: 10.1021/bi00391a004. [DOI] [PubMed] [Google Scholar]
- Schullek J. R., Ruf W., Edgington T. S. Key ligand interface residues in tissue factor contribute independently to factor VIIa binding. J Biol Chem. 1994 Jul 29;269(30):19399–19403. [PubMed] [Google Scholar]
- Shigematsu Y., Miyata T., Higashi S., Miki T., Sadler J. E., Iwanaga S. Expression of human soluble tissue factor in yeast and enzymatic properties of its complex with factor VIIa. J Biol Chem. 1992 Oct 25;267(30):21329–21337. [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Snaith S. M., Levvy G. A. Purification and properties of alpha-D-mannosidase from jack-bean meal. Biochem J. 1968 Dec;110(4):663–670. doi: 10.1042/bj1100663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicer E. K., Horton R., Bloem L., Bach R., Williams K. R., Guha A., Kraus J., Lin T. C., Nemerson Y., Konigsberg W. H. Isolation of cDNA clones coding for human tissue factor: primary structure of the protein and cDNA. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5148–5152. doi: 10.1073/pnas.84.15.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor F. B., Jr, Chang A., Ruf W., Morrissey J. H., Hinshaw L., Catlett R., Blick K., Edgington T. S. Lethal E. coli septic shock is prevented by blocking tissue factor with monoclonal antibody. Circ Shock. 1991 Mar;33(3):127–134. [PubMed] [Google Scholar]
- Tsai P. K., Frevert J., Ballou C. E. Carbohydrate structure of Saccharomyces cerevisiae mnn9 mannoprotein. J Biol Chem. 1984 Mar 25;259(6):3805–3811. [PubMed] [Google Scholar]
- Yang J. T., Wu C. S., Martinez H. M. Calculation of protein conformation from circular dichroism. Methods Enzymol. 1986;130:208–269. doi: 10.1016/0076-6879(86)30013-2. [DOI] [PubMed] [Google Scholar]