Abstract
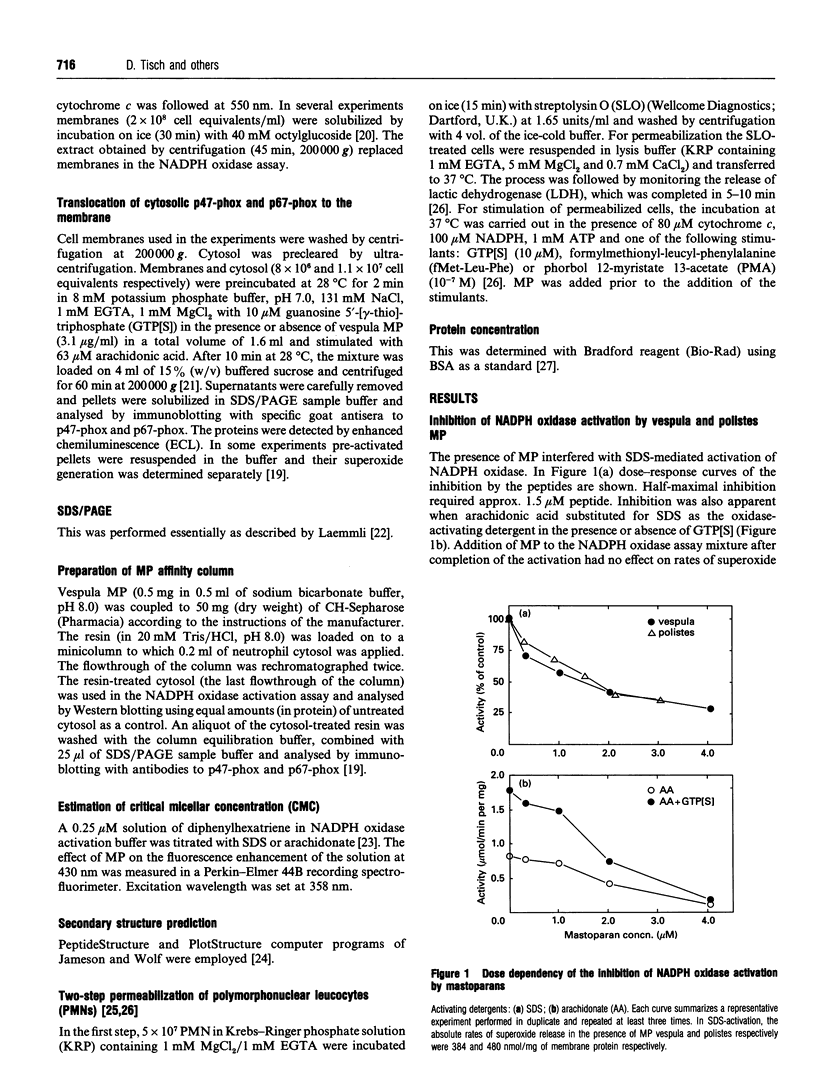
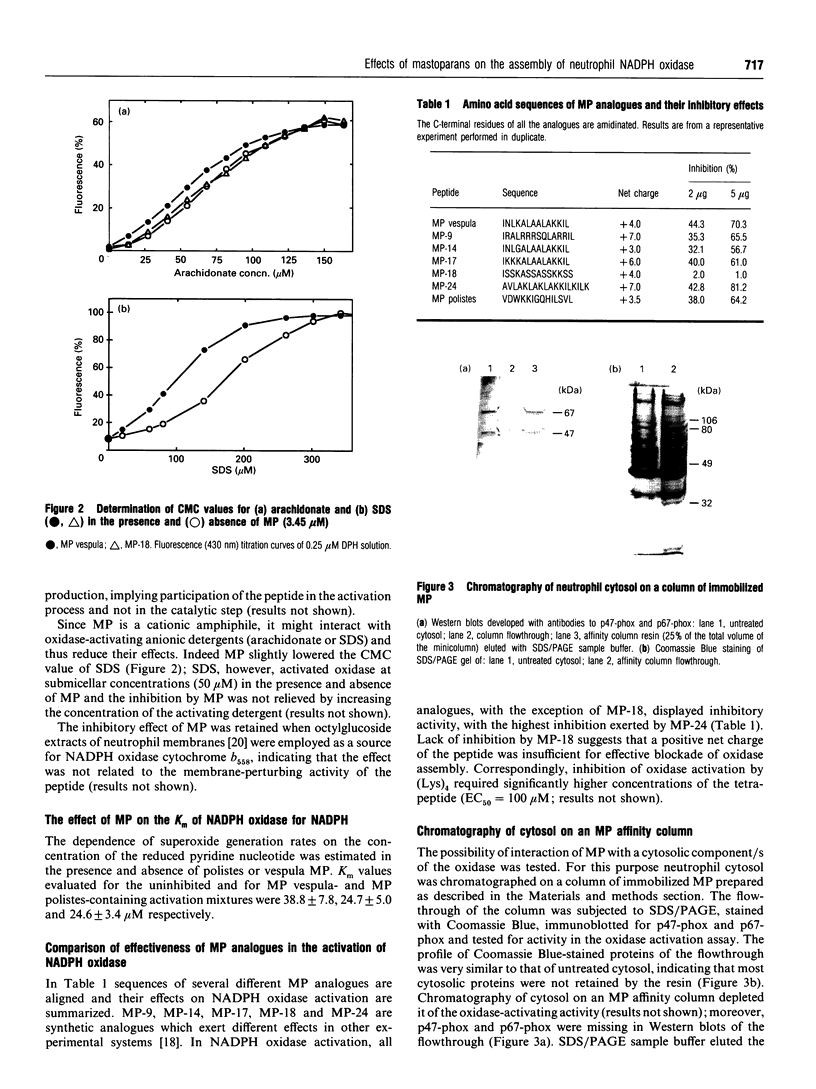
Detergent-mediated activation of the phagocyte superoxide-generating NADPH oxidase requires the participation of at least four proteins: the membrane-bound heterodimeric cytochrome b558 and three cytosolic components, p47-phox, p67-phox and a Rac1/Rac2 protein. Peptides corresponding to sequences of different subunits of NADPH oxidase have been used as probes of the mechanism and sequence of assembly of the active complex. In the present study effects of mastoparans on activation of NADPH oxidase were investigated. Mastoparans are wasp venom cationic amphiphilic tetradecapeptides capable of modulation of various cellular activities. Natural mastoparans, as well as several synthetic mastoparan analogues, unrelated to oxidase components, blocked activation of the oxidase in the cell-free system (EC50 = 1.5 microM) and in guanosine 5'-[gamma-thio]triphosphate (GTP[S])/ATP-stimulated neutrophils permeabilized with streptolysin O. In the cell-free system the effect was not relieved by raising the detergent concentration and could not be ascribed to changes in critical micellar concentration values of the activating SDS or arachidonate. Chromatography of neutrophil cytosol on an immobilized mastoparan column suggested interaction of cytosolic p47-phox and p67-phox with the peptide. In spite of this interaction mastoparan did not interfere with translocation of p47-phox and p67-phox to the cell membranes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo A., Boyhan A., West I., Thrasher A. J., Segal A. W. Reconstitution of neutrophil NADPH oxidase activity in the cell-free system by four components: p67-phox, p47-phox, p21rac1, and cytochrome b-245. J Biol Chem. 1992 Aug 25;267(24):16767–16770. [PubMed] [Google Scholar]
- Abo A., Webb M. R., Grogan A., Segal A. W. Activation of NADPH oxidase involves the dissociation of p21rac from its inhibitory GDP/GTP exchange protein (rhoGDI) followed by its translocation to the plasma membrane. Biochem J. 1994 Mar 15;298(Pt 3):585–591. doi: 10.1042/bj2980585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argiolas A., Pisano J. J. Facilitation of phospholipase A2 activity by mastoparans, a new class of mast cell degranulating peptides from wasp venom. J Biol Chem. 1983 Nov 25;258(22):13697–13702. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Volpp B. D., Leidal K. G., Nauseef W. M. Two cytosolic components of the human neutrophil respiratory burst oxidase translocate to the plasma membrane during cell activation. J Clin Invest. 1990 Mar;85(3):714–721. doi: 10.1172/JCI114496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilenko M., Worland P., Carlson B., Sausville E. A., Sharoni Y. Selective effects of mastoparan analogs: separation of G-protein-directed and membrane-perturbing activities. Biochem Biophys Res Commun. 1993 Nov 15;196(3):1296–1302. doi: 10.1006/bbrc.1993.2393. [DOI] [PubMed] [Google Scholar]
- Fensome A., Cunningham E., Troung O., Cockcroft S. ARF1(2-17) does not specifically interact with ARF1-dependent pathways. Inhibition by peptide of phospholipases C beta, D and exocytosis in HL60 cells. FEBS Lett. 1994 Jul 25;349(1):34–38. doi: 10.1016/0014-5793(94)00634-2. [DOI] [PubMed] [Google Scholar]
- Gravotta D., Adesnik M., Sabatini D. D. Transport of influenza HA from the trans-Golgi network to the apical surface of MDCK cells permeabilized in their basolateral plasma membranes: energy dependence and involvement of GTP-binding proteins. J Cell Biol. 1990 Dec;111(6 Pt 2):2893–2908. doi: 10.1083/jcb.111.6.2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashijima T., Uzu S., Nakajima T., Ross E. M. Mastoparan, a peptide toxin from wasp venom, mimics receptors by activating GTP-binding regulatory proteins (G proteins). J Biol Chem. 1988 May 15;263(14):6491–6494. [PubMed] [Google Scholar]
- Jameson B. A., Wolf H. The antigenic index: a novel algorithm for predicting antigenic determinants. Comput Appl Biosci. 1988 Mar;4(1):181–186. doi: 10.1093/bioinformatics/4.1.181. [DOI] [PubMed] [Google Scholar]
- Joseph G., Gorzalczany Y., Koshkin V., Pick E. Inhibition of NADPH oxidase activation by synthetic peptides mapping within the carboxyl-terminal domain of small GTP-binding proteins. Lack of amino acid sequence specificity and importance of polybasic motif. J Biol Chem. 1994 Nov 18;269(46):29024–29031. [PubMed] [Google Scholar]
- Klinger E., Sharabani M., Aviram I. The interaction of cytosolic components of neutrophil NADPH oxidase with phosphoinositides. Biochem J. 1993 Oct 15;295(Pt 2):565–570. doi: 10.1042/bj2950565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoller S., Shpungin S., Pick E. The membrane-associated component of the amphiphile-activated, cytosol-dependent superoxide-forming NADPH oxidase of macrophages is identical to cytochrome b559. J Biol Chem. 1991 Feb 15;266(5):2795–2804. [PubMed] [Google Scholar]
- Koch G., Haberman B., Mohr C., Just I., Aktories K. Interaction of mastoparan with the low molecular mass GTP-binding proteins rho/rac. FEBS Lett. 1991 Oct 21;291(2):336–340. doi: 10.1016/0014-5793(91)81315-y. [DOI] [PubMed] [Google Scholar]
- Kreck M. L., Uhlinger D. J., Tyagi S. R., Inge K. L., Lambeth J. D. Participation of the small molecular weight GTP-binding protein Rac1 in cell-free activation and assembly of the respiratory burst oxidase. Inhibition by a carboxyl-terminal Rac peptide. J Biol Chem. 1994 Feb 11;269(6):4161–4168. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lu D. J., Grinstein S. ATP and guanine nucleotide dependence of neutrophil activation. Evidence for the involvement of two distinct GTP-binding proteins. J Biol Chem. 1990 Aug 15;265(23):13721–13729. [PubMed] [Google Scholar]
- Malencik D. A., Anderson S. R. High affinity binding of the mastoparans by calmodulin. Biochem Biophys Res Commun. 1983 Jul 18;114(1):50–56. doi: 10.1016/0006-291x(83)91592-9. [DOI] [PubMed] [Google Scholar]
- Morel F., Doussiere J., Vignais P. V. The superoxide-generating oxidase of phagocytic cells. Physiological, molecular and pathological aspects. Eur J Biochem. 1991 Nov 1;201(3):523–546. doi: 10.1111/j.1432-1033.1991.tb16312.x. [DOI] [PubMed] [Google Scholar]
- Nakanishi A., Imajoh-Ohmi S., Fujinawa T., Kikuchi H., Kanegasaki S. Direct evidence for interaction between COOH-terminal regions of cytochrome b558 subunits and cytosolic 47-kDa protein during activation of an O(2-)-generating system in neutrophils. J Biol Chem. 1992 Sep 25;267(27):19072–19074. [PubMed] [Google Scholar]
- Nauseef W. M., McCormick S., Renee J., Leidal K. G., Clark R. A. Functional domain in an arginine-rich carboxyl-terminal region of p47phox. J Biol Chem. 1993 Nov 5;268(31):23646–23651. [PubMed] [Google Scholar]
- Okano Y., Takagi H., Tohmatsu T., Nakashima S., Kuroda Y., Saito K., Nozawa Y. A wasp venom mastoparan-induced polyphosphoinositide breakdown in rat peritoneal mast cells. FEBS Lett. 1985 Sep 2;188(2):363–366. doi: 10.1016/0014-5793(85)80403-8. [DOI] [PubMed] [Google Scholar]
- Park J. W., Babior B. M. The translocation of respiratory burst oxidase components from cytosol to plasma membrane is regulated by guanine nucleotides and diacylglycerol. J Biol Chem. 1992 Oct 5;267(28):19901–19906. [PubMed] [Google Scholar]
- Park J. W., Benna J. E., Scott K. E., Christensen B. L., Chanock S. J., Babior B. M. Isolation of a complex of respiratory burst oxidase components from resting neutrophil cytosol. Biochemistry. 1994 Mar 15;33(10):2907–2911. doi: 10.1021/bi00176a021. [DOI] [PubMed] [Google Scholar]
- Rotrosen D., Kleinberg M. E., Nunoi H., Leto T., Gallin J. I., Malech H. L. Evidence for a functional cytoplasmic domain of phagocyte oxidase cytochrome b558. J Biol Chem. 1990 May 25;265(15):8745–8750. [PubMed] [Google Scholar]
- Rotrosen D., Yeung C. L., Katkin J. P. Production of recombinant cytochrome b558 allows reconstitution of the phagocyte NADPH oxidase solely from recombinant proteins. J Biol Chem. 1993 Jul 5;268(19):14256–14260. [PubMed] [Google Scholar]
- Sawai T., Asada M., Nunoi H., Matsuda I., Ando S., Sasaki T., Kaibuchi K., Takai Y., Katayama K. Combination of arachidonic acid and guanosine 5'-O-(3-thiotriphosphate) induce translocation of rac p21s to membrane and activation of NADPH oxidase in a cell-free system. Biochem Biophys Res Commun. 1993 Aug 31;195(1):264–269. doi: 10.1006/bbrc.1993.2039. [DOI] [PubMed] [Google Scholar]
- Segal A. W., Abo A. The biochemical basis of the NADPH oxidase of phagocytes. Trends Biochem Sci. 1993 Feb;18(2):43–47. doi: 10.1016/0968-0004(93)90051-n. [DOI] [PubMed] [Google Scholar]
- Serth J., Lautwein A., Frech M., Wittinghofer A., Pingoud A. The inhibition of the GTPase activating protein-Ha-ras interaction by acidic lipids is due to physical association of the C-terminal domain of the GTPase activating protein with micellar structures. EMBO J. 1991 Jun;10(6):1325–1330. doi: 10.1002/j.1460-2075.1991.tb07651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal T., Aviram I. Defensin interferes with the activation of neutrophil NADPH oxidase in a cell-free system. Biochem Biophys Res Commun. 1993 Oct 29;196(2):636–641. doi: 10.1006/bbrc.1993.2297. [DOI] [PubMed] [Google Scholar]
- Tyagi S. R., Neckelmann N., Uhlinger D. J., Burnham D. N., Lambeth J. D. Cell-free translocation of recombinant p47-phox, a component of the neutrophil NADPH oxidase: effects of guanosine 5'-O-(3-thiotriphosphate), diacylglycerol, and an anionic amphiphile. Biochemistry. 1992 Mar 17;31(10):2765–2774. doi: 10.1021/bi00125a017. [DOI] [PubMed] [Google Scholar]