Abstract
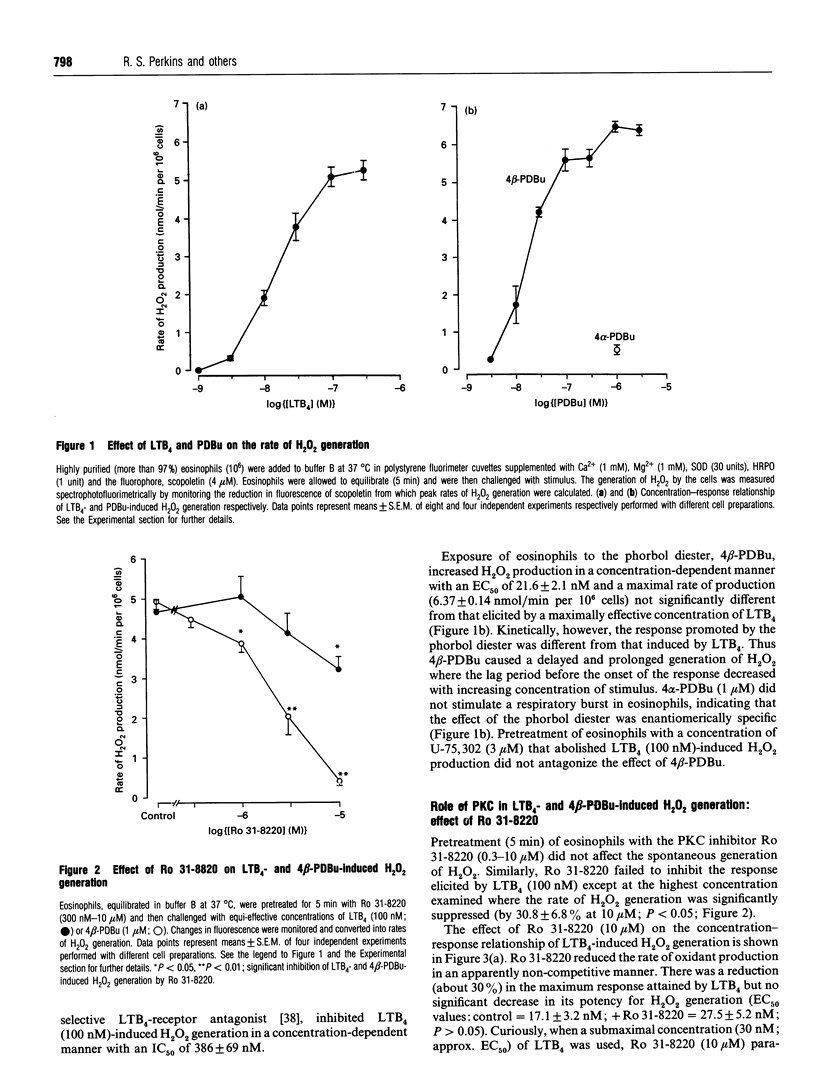
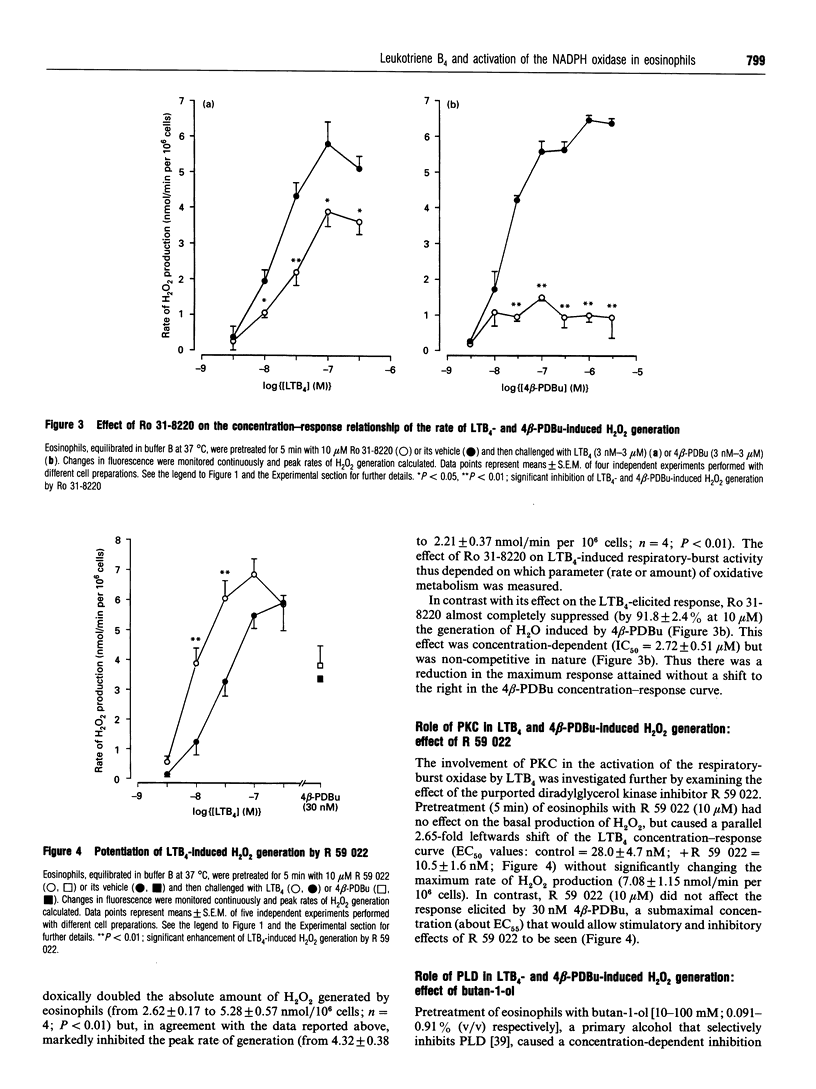
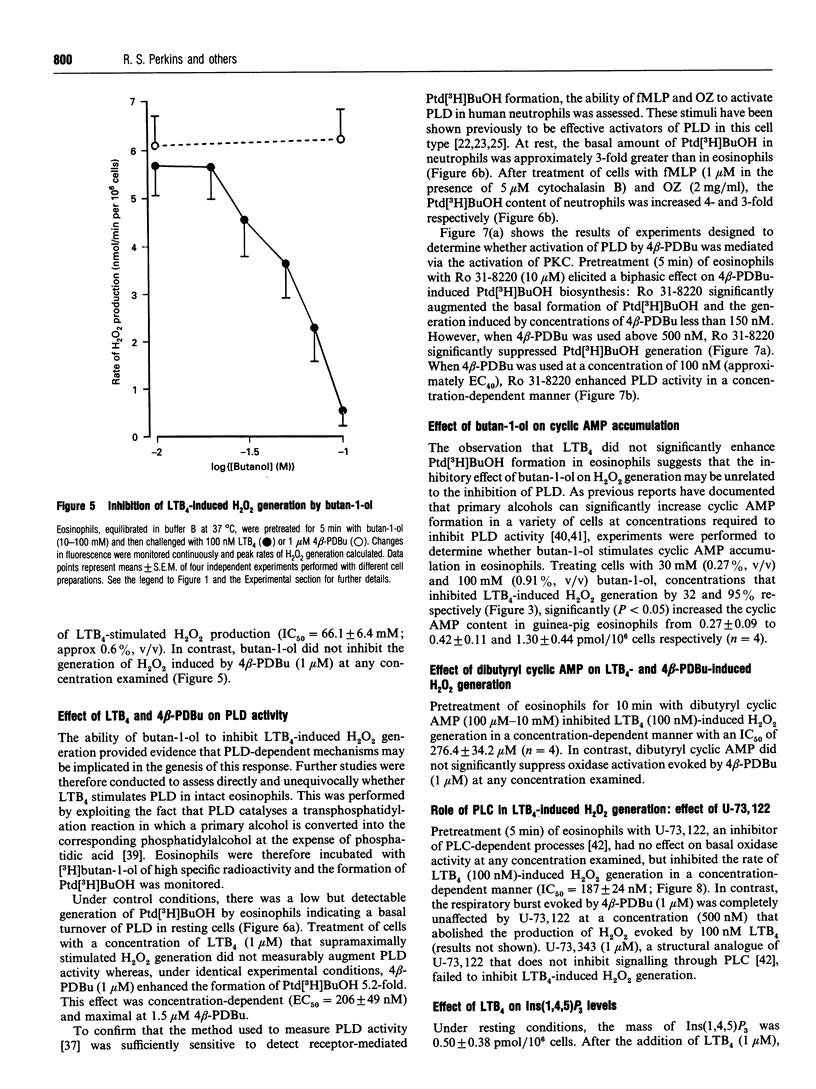
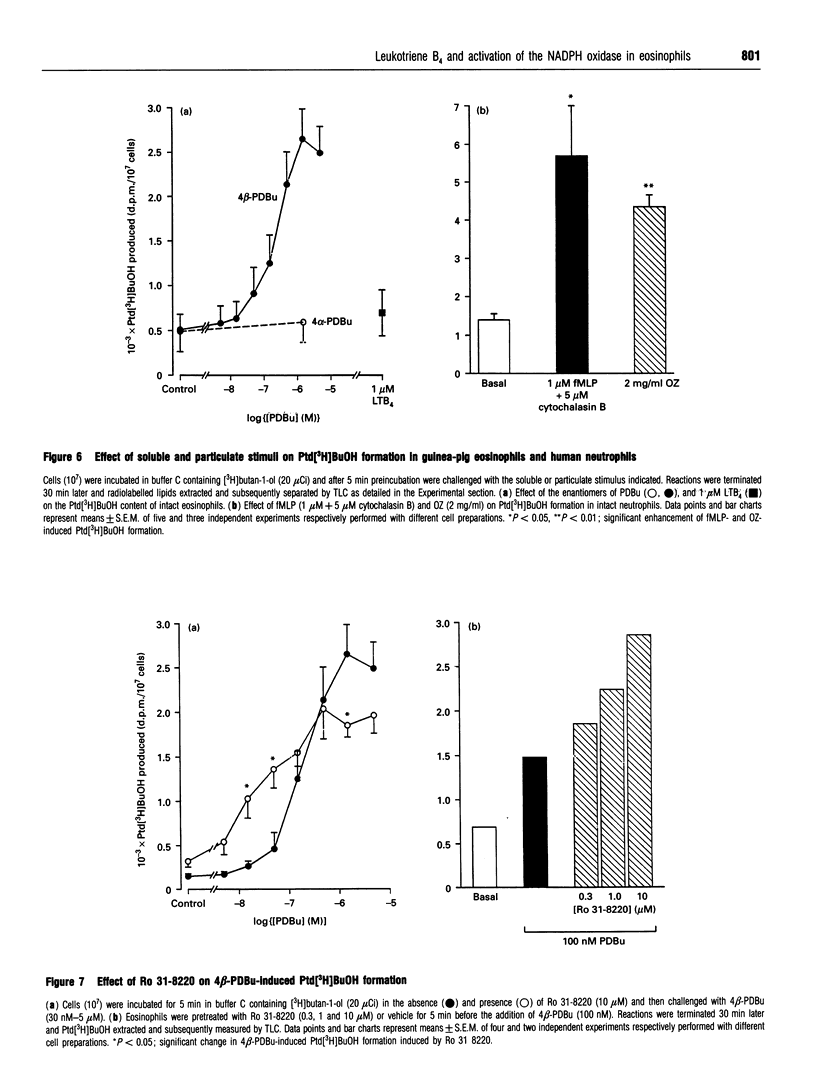
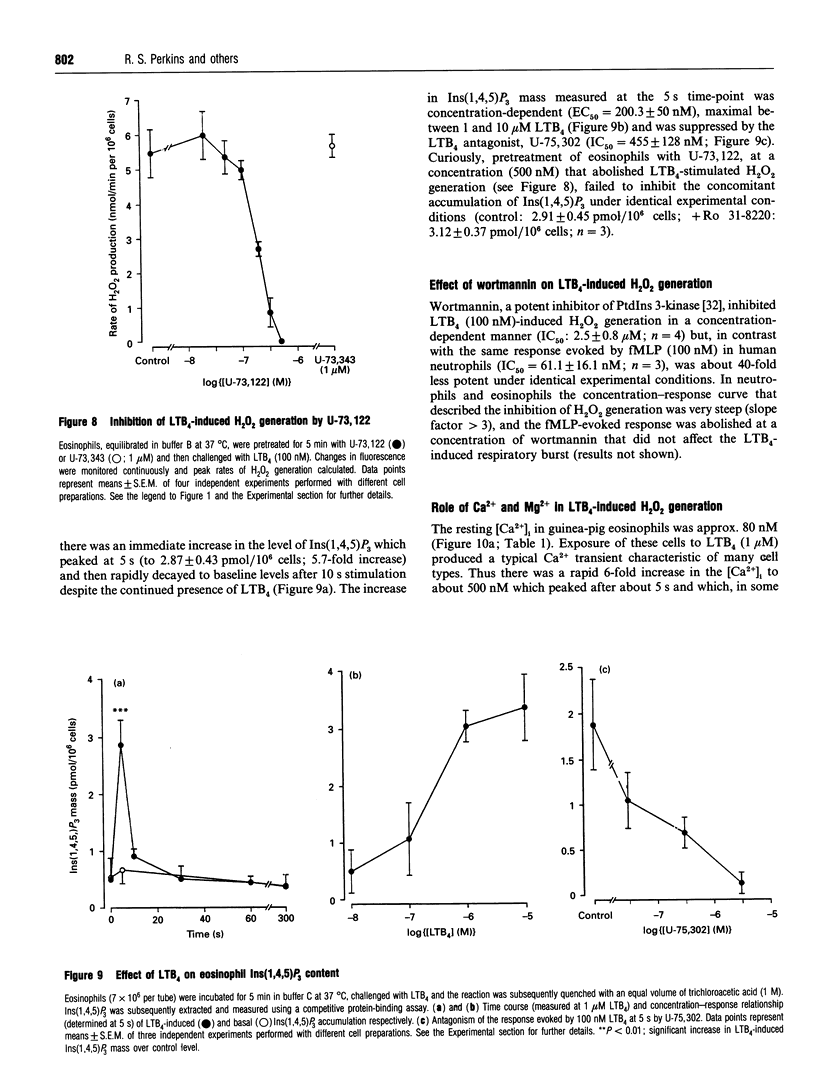
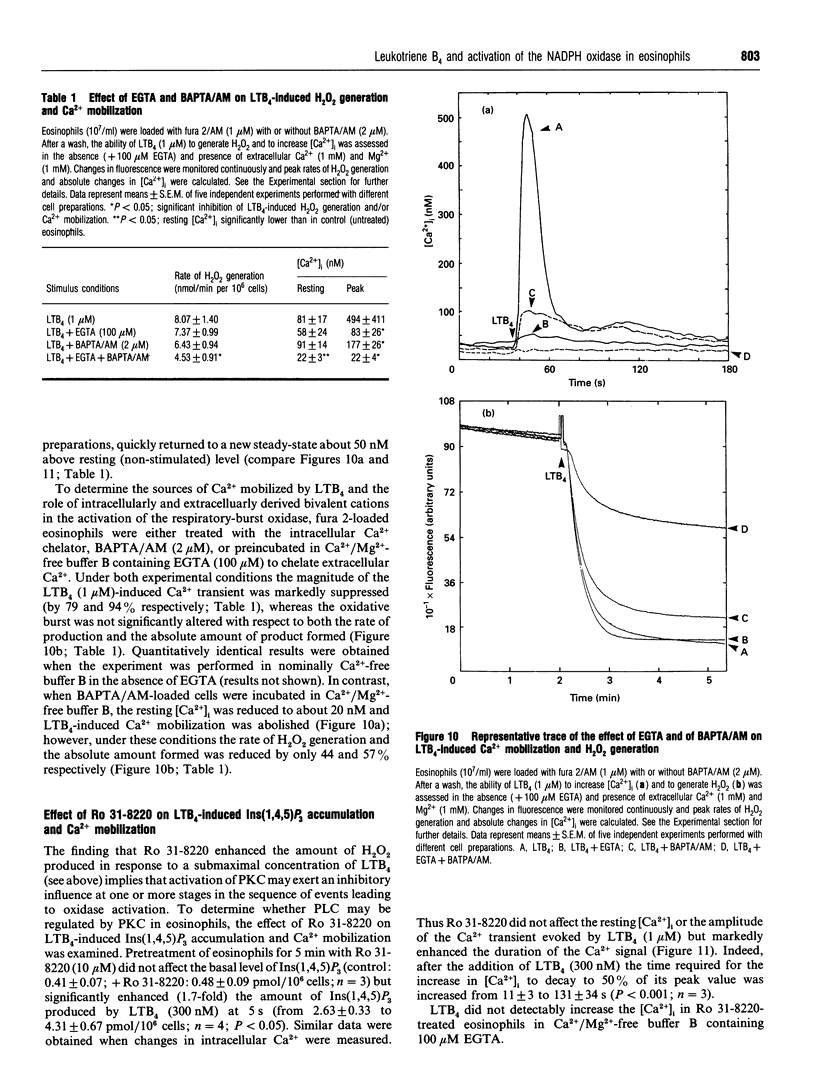
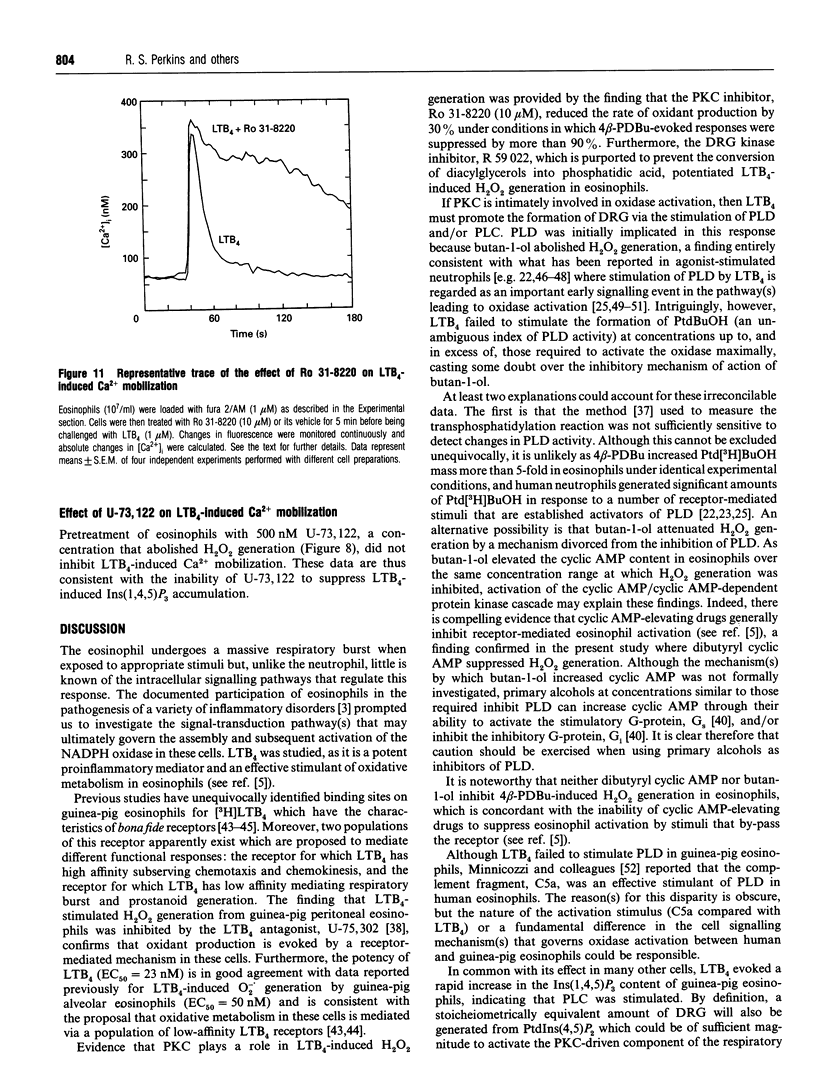
The early signalling events that may ultimately contribute to the assembly and subsequent activation of the NADPH oxidase in guinea-pig peritoneal eosinophils were investigated in response to leukotriene B4 (LTB4). LTB4 promoted a rapid, transient and receptor-mediated increase in the rate of H2O2 generation that was potentiated by R 59 022, a diradylglycerol (DRG) kinase inhibitor, implicating protein kinase C (PKC) in the genesis of this response. This conclusion was supported by the finding that the PKC inhibitor, Ro 31-8220, attenuated (by about 30%) the peak rate of LTB4-induced H2O2 generation under conditions where the same response evoked by 4 beta-phorbol 12,13-dibutyrate (PDBu) was inhibited by more than 90%. Paradoxically, Ro 31-8220 doubled the amount of H2O2 produced by LTB4 which may relate to the ability of PKC to inhibit cell signalling through phospholipase C (PLC). Indeed, Ro 31-8220 significantly enhanced LTB4-induced Ins(1,4,5)P3 accumulation and the duration of the Ca2+ transient in eosinophils. Experiments designed to assess the relative importance of DRG-mobilizing phospholipases in LTB4-induced oxidase activation indicated that phospholipase D (PLD) did not play a major role. Thus, although H2O2 generation was abolished by butan-1-ol, this was apparently unrelated to the inhibition of PLD, as LTB4 failed to stimulate the formation of Ptd[3H]BuOH in [3H]butan-1-ol-treated eosinophils. Rather, the inhibition was probably due to the ability of butan-1-ol to increase the eosinophil cyclic AMP content. In contrast, Ca(2+)- and PLC-driven mechanisms were implicated in H2O2 generation, as LTB4 elevated the Ins(1,4,5)P3 content and intracellular free Ca2+ concentration in intact cells, and cochelation of extracellular and intracellular Ca2+ significantly attenuated LTB4-induced H2O2 generation. Pretreatment of eosinophils with wortmannin did not affect LTB4-induced H2O2 production at concentrations at which it abolished the respiratory burst evoked by formylmethionyl-leucylphenylalanine in human neutrophils. Collectively, these data suggest that LTB4 activates the NADPH oxidase in eosinophils by PLD- and PtdIns 3-kinase-independent mechanisms that involve Ca2+, PLC and PKC. Furthermore, the activation of additional pathways that do not require Ca2+ is also suggested by the finding that LTB4 evoked a significant respiratory burst in Ca(2+)-depleted cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebischer C. P., Pasche I., Jörg A. Nanomolar arachidonic acid influences the respiratory burst in eosinophils and neutrophils induced by GTP-binding protein. A comparative study of the respiratory burst in bovine eosinophils and neutrophils. Eur J Biochem. 1993 Dec 1;218(2):669–677. doi: 10.1111/j.1432-1033.1993.tb18421.x. [DOI] [PubMed] [Google Scholar]
- Agosti J. M., Altman L. C., Ayars G. H., Loegering D. A., Gleich G. J., Klebanoff S. J. The injurious effect of eosinophil peroxidase, hydrogen peroxide, and halides on pneumocytes in vitro. J Allergy Clin Immunol. 1987 Mar;79(3):496–504. doi: 10.1016/0091-6749(87)90368-x. [DOI] [PubMed] [Google Scholar]
- Agwu D. E., McCall C. E., McPhail L. C. Regulation of phospholipase D-induced hydrolysis of choline-containing phosphoglycerides by cyclic AMP in human neutrophils. J Immunol. 1991 Jun 1;146(11):3895–3903. [PubMed] [Google Scholar]
- Arcaro A., Wymann M. P. Wortmannin is a potent phosphatidylinositol 3-kinase inhibitor: the role of phosphatidylinositol 3,4,5-trisphosphate in neutrophil responses. Biochem J. 1993 Dec 1;296(Pt 2):297–301. doi: 10.1042/bj2960297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austen K. F., Orange R. P. Bronchial asthma: the possible role of the chemical mediators of immediate hypersensitivity in the pathogenesis of subacute chronic disease. Am Rev Respir Dis. 1975 Sep;112(3):423–436. doi: 10.1164/arrd.1975.112.3.423. [DOI] [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billah M. M., Anthes J. C. The regulation and cellular functions of phosphatidylcholine hydrolysis. Biochem J. 1990 Jul 15;269(2):281–291. doi: 10.1042/bj2690281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleasdale J. E., Thakur N. R., Gremban R. S., Bundy G. L., Fitzpatrick F. A., Smith R. J., Bunting S. Selective inhibition of receptor-coupled phospholipase C-dependent processes in human platelets and polymorphonuclear neutrophils. J Pharmacol Exp Ther. 1990 Nov;255(2):756–768. [PubMed] [Google Scholar]
- Bolscher B. G., Koenderman L., Tool A. T., Stokman P. M., Roos D. NADPH:O2 oxidoreductase of human eosinophils in the cell-free system. FEBS Lett. 1990 Jul 30;268(1):269–273. doi: 10.1016/0014-5793(90)81025-j. [DOI] [PubMed] [Google Scholar]
- Bonser R. W., Thompson N. T., Randall R. W., Garland L. G. Phospholipase D activation is functionally linked to superoxide generation in the human neutrophil. Biochem J. 1989 Dec 1;264(2):617–620. doi: 10.1042/bj2640617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth A. E., Wassom D. L., Gleich G. J., Loegering D. A., David J. R. Damage to schistosomula of Schistosoma mansoni induced directly by eosinophil major basic protein. J Immunol. 1979 Jan;122(1):221–229. [PubMed] [Google Scholar]
- Challiss R. A., Batty I. H., Nahorski S. R. Mass measurements of inositol(1,4,5)trisphosphate in rat cerebral cortex slices using a radioreceptor assay: effects of neurotransmitters and depolarization. Biochem Biophys Res Commun. 1988 Dec 15;157(2):684–691. doi: 10.1016/s0006-291x(88)80304-8. [DOI] [PubMed] [Google Scholar]
- Connolly T. M., Lawing W. J., Jr, Majerus P. W. Protein kinase C phosphorylates human platelet inositol trisphosphate 5'-phosphomonoesterase, increasing the phosphatase activity. Cell. 1986 Sep 12;46(6):951–958. doi: 10.1016/0092-8674(86)90077-2. [DOI] [PubMed] [Google Scholar]
- Dana R., Malech H. L., Levy R. The requirement for phospholipase A2 for activation of the assembled NADPH oxidase in human neutrophils. Biochem J. 1994 Jan 1;297(Pt 1):217–223. doi: 10.1042/bj2970217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Hawkins P. T., Irvine R. F. Inositol 1,3,4,5-tetrakisphosphate and not phosphatidylinositol 3,4-bisphosphate is the probable precursor of inositol 1,3,4-trisphosphate in agonist-stimulated parotid gland. Biochem J. 1986 Sep 1;238(2):501–506. doi: 10.1042/bj2380501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gärtner I. Separation of human eosinophils in density gradients of polyvinylpyrrolidone-coated silica gel (Percoll). Immunology. 1980 May;40(1):133–136. [PMC free article] [PubMed] [Google Scholar]
- Henderson L. M., Chappell J. B., Jones O. T. Superoxide generation is inhibited by phospholipase A2 inhibitors. Role for phospholipase A2 in the activation of the NADPH oxidase. Biochem J. 1989 Nov 15;264(1):249–255. doi: 10.1042/bj2640249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson W. R., Chi E. Y., Klebanoff S. J. Eosinophil peroxidase-induced mast cell secretion. J Exp Med. 1980 Aug 1;152(2):265–279. doi: 10.1084/jem.152.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P. L., Tabakoff B. Ethanol and guanine nucleotide binding proteins: a selective interaction. FASEB J. 1990 Jun;4(9):2612–2622. doi: 10.1096/fasebj.4.9.2161371. [DOI] [PubMed] [Google Scholar]
- Kanaho Y., Kanoh H., Saitoh K., Nozawa Y. Phospholipase D activation by platelet-activating factor, leukotriene B4, and formyl-methionyl-leucyl-phenylalanine in rabbit neutrophils. Phospholipase D activation is involved in enzyme release. J Immunol. 1991 May 15;146(10):3536–3541. [PubMed] [Google Scholar]
- Kessels G. C., Krause K. H., Verhoeven A. J. Protein kinase C activity is not involved in N-formylmethionyl-leucyl-phenylalanine-induced phospholipase D activation in human neutrophils, but is essential for concomitant NADPH oxidase activation: studies with a staurosporine analogue with improved selectivity for protein kinase C. Biochem J. 1993 Jun 15;292(Pt 3):781–785. doi: 10.1042/bj2920781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels G. C., Roos D., Verhoeven A. J. fMet-Leu-Phe-induced activation of phospholipase D in human neutrophils. Dependence on changes in cytosolic free Ca2+ concentration and relation with respiratory burst activation. J Biol Chem. 1991 Dec 5;266(34):23152–23156. [PubMed] [Google Scholar]
- Lawson C. F., Wishka D. G., Morris J., Fitzpatrick F. A. Receptor antagonism of leukotriene B4 myotropic activity by the 2,6 disubstituted pyridine analog U-75302: characterization on lung parenchyma strips. J Lipid Mediat. 1989 Jan-Feb;1(1):3–12. [PubMed] [Google Scholar]
- Maghni K., de Brum-Fernandes A. J., Földes-Filep E., Gaudry M., Borgeat P., Sirois P. Leukotriene B4 receptors on guinea pig alveolar eosinophils. J Pharmacol Exp Ther. 1991 Sep;258(3):784–789. [PubMed] [Google Scholar]
- Mayeno A. N., Curran A. J., Roberts R. L., Foote C. S. Eosinophils preferentially use bromide to generate halogenating agents. J Biol Chem. 1989 Apr 5;264(10):5660–5668. [PubMed] [Google Scholar]
- McPhail L. C., Clayton C. C., Snyderman R. The NADPH oxidase of human polymorphonuclear leukocytes. Evidence for regulation by multiple signals. J Biol Chem. 1984 May 10;259(9):5768–5775. [PubMed] [Google Scholar]
- Meyer T., Holowka D., Stryer L. Highly cooperative opening of calcium channels by inositol 1,4,5-trisphosphate. Science. 1988 Apr 29;240(4852):653–656. doi: 10.1126/science.2452482. [DOI] [PubMed] [Google Scholar]
- Minnicozzi M., Anthes J. C., Siegel M. I., Billah M. M., Egan R. W. Activation of phospholipase D in normodense human eosinophils. Biochem Biophys Res Commun. 1990 Jul 31;170(2):540–547. doi: 10.1016/0006-291x(90)92125-j. [DOI] [PubMed] [Google Scholar]
- Molina y Vedia L. M., Lapetina E. G. Phorbol 12,13-dibutyrate and 1-oleyl-2-acetyldiacylglycerol stimulate inositol trisphosphate dephosphorylation in human platelets. J Biol Chem. 1986 Aug 15;261(23):10493–10495. [PubMed] [Google Scholar]
- Morel F., Doussiere J., Vignais P. V. The superoxide-generating oxidase of phagocytic cells. Physiological, molecular and pathological aspects. Eur J Biochem. 1991 Nov 1;201(3):523–546. doi: 10.1111/j.1432-1033.1991.tb16312.x. [DOI] [PubMed] [Google Scholar]
- Motojima S., Frigas E., Loegering D. A., Gleich G. J. Toxicity of eosinophil cationic proteins for guinea pig tracheal epithelium in vitro. Am Rev Respir Dis. 1989 Mar;139(3):801–805. doi: 10.1164/ajrccm/139.3.801. [DOI] [PubMed] [Google Scholar]
- Nagy L. E., DeSilva S. E. Ethanol increases receptor-dependent cyclic AMP production in cultured hepatocytes by decreasing G(i)-mediated inhibition. Biochem J. 1992 Sep 15;286(Pt 3):681–686. doi: 10.1042/bj2860681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng C. F., Sun F. F., Taylor B. M., Wolin M. S., Wong P. Y. Functional properties of guinea pig eosinophil leukotriene B4 receptor. J Immunol. 1991 Nov 1;147(9):3096–3103. [PubMed] [Google Scholar]
- O'Flaherty J. T., Rossi A. G., Jacobson D. P., Redman J. F. Roles of Ca2+ in human neutrophil responses to receptor agonists. Biochem J. 1991 Aug 1;277(Pt 3):705–711. doi: 10.1042/bj2770705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry D. K., Hand W. L., Edmondson D. E., Lambeth J. D. Role of phospholipase D-derived diradylglycerol in the activation of the human neutrophil respiratory burst oxidase. Inhibition by phosphatidic acid phosphohydrolase inhibitors. J Immunol. 1992 Oct 15;149(8):2749–2758. [PubMed] [Google Scholar]
- Petreccia D. C., Nauseef W. M., Clark R. A. Respiratory burst of normal human eosinophils. J Leukoc Biol. 1987 Apr;41(4):283–288. doi: 10.1002/jlb.41.4.283. [DOI] [PubMed] [Google Scholar]
- Qualliotine-Mann D., Agwu D. E., Ellenburg M. D., McCall C. E., McPhail L. C. Phosphatidic acid and diacylglycerol synergize in a cell-free system for activation of NADPH oxidase from human neutrophils. J Biol Chem. 1993 Nov 15;268(32):23843–23849. [PubMed] [Google Scholar]
- Randall R. W., Bonser R. W., Thompson N. T., Garland L. G. A novel and sensitive assay for phospholipase D in intact cells. FEBS Lett. 1990 May 7;264(1):87–90. doi: 10.1016/0014-5793(90)80772-b. [DOI] [PubMed] [Google Scholar]
- Reinhold S. L., Prescott S. M., Zimmerman G. A., McIntyre T. M. Activation of human neutrophil phospholipase D by three separable mechanisms. FASEB J. 1990 Feb 1;4(2):208–214. doi: 10.1096/fasebj.4.2.2105252. [DOI] [PubMed] [Google Scholar]
- Root R. K., Metcalf J., Oshino N., Chance B. H2O2 release from human granulocytes during phagocytosis. I. Documentation, quantitation, and some regulating factors. J Clin Invest. 1975 May;55(5):945–955. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu S. H., Kim U. H., Wahl M. I., Brown A. B., Carpenter G., Huang K. P., Rhee S. G. Feedback regulation of phospholipase C-beta by protein kinase C. J Biol Chem. 1990 Oct 15;265(29):17941–17945. [PubMed] [Google Scholar]
- Sedgwick J. B., Vrtis R. F., Gourley M. F., Busse W. W. Stimulus-dependent differences in superoxide anion generation by normal human eosinophils and neutrophils. J Allergy Clin Immunol. 1988 May;81(5 Pt 1):876–883. doi: 10.1016/0091-6749(88)90945-1. [DOI] [PubMed] [Google Scholar]
- Segal A. W., Abo A. The biochemical basis of the NADPH oxidase of phagocytes. Trends Biochem Sci. 1993 Feb;18(2):43–47. doi: 10.1016/0968-0004(93)90051-n. [DOI] [PubMed] [Google Scholar]
- Sehmi R., Rossi A. G., Kay A. B., Cromwell O. Identification on receptors for leukotriene B4 expressed on guinea-pig peritoneal eosinophils. Immunology. 1992 Sep;77(1):129–135. [PMC free article] [PubMed] [Google Scholar]
- Smith R. J., Sam L. M., Justen J. M., Bundy G. L., Bala G. A., Bleasdale J. E. Receptor-coupled signal transduction in human polymorphonuclear neutrophils: effects of a novel inhibitor of phospholipase C-dependent processes on cell responsiveness. J Pharmacol Exp Ther. 1990 May;253(2):688–697. [PubMed] [Google Scholar]
- Spät A., Bradford P. G., McKinney J. S., Rubin R. P., Putney J. W., Jr A saturable receptor for 32P-inositol-1,4,5-triphosphate in hepatocytes and neutrophils. Nature. 1986 Feb 6;319(6053):514–516. doi: 10.1038/319514a0. [DOI] [PubMed] [Google Scholar]
- Suchard S. J., Nakamura T., Abe A., Shayman J. A., Boxer L. A. Phospholipase D-mediated diradylglycerol formation coincides with H2O2 and lactoferrin release in adherent human neutrophils. J Biol Chem. 1994 Mar 18;269(11):8063–8068. [PubMed] [Google Scholar]
- Tauber A. I., Goetzl E. J., Babior B. M. Unique characteristics of superoxide production by human eosinophils in eosinophilic states. Inflammation. 1979 Jul;3(3):261–272. doi: 10.1007/BF00914183. [DOI] [PubMed] [Google Scholar]
- Thompson N. T., Tateson J. E., Randall R. W., Spacey G. D., Bonser R. W., Garland L. G. The temporal relationship between phospholipase activation, diradylglycerol formation and superoxide production in the human neutrophil. Biochem J. 1990 Oct 1;271(1):209–213. doi: 10.1042/bj2710209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uings I. J., Thompson N. T., Randall R. W., Spacey G. D., Bonser R. W., Hudson A. T., Garland L. G. Tyrosine phosphorylation is involved in receptor coupling to phospholipase D but not phospholipase C in the human neutrophil. Biochem J. 1992 Feb 1;281(Pt 3):597–600. doi: 10.1042/bj2810597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., Test S. T., Eckmann C. M., Roos D., Regiani S. Brominating oxidants generated by human eosinophils. Science. 1986 Oct 10;234(4773):200–203. doi: 10.1126/science.3018933. [DOI] [PubMed] [Google Scholar]
- Yamashita T., Someya A., Hara E. Response of superoxide anion production by guinea pig eosinophils to various soluble stimuli: comparison to neutrophils. Arch Biochem Biophys. 1985 Sep;241(2):447–452. doi: 10.1016/0003-9861(85)90569-7. [DOI] [PubMed] [Google Scholar]
- Yang S. F., Freer S., Benson A. A. Transphosphatidylation by phospholipase D. J Biol Chem. 1967 Feb 10;242(3):477–484. [PubMed] [Google Scholar]
- Zhou H. L., Chabot-Fletcher M., Foley J. J., Sarau H. M., Tzimas M. N., Winkler J. D., Torphy T. J. Association between leukotriene B4-induced phospholipase D activation and degranulation of human neutrophils. Biochem Pharmacol. 1993 Jul 6;46(1):139–148. doi: 10.1016/0006-2952(93)90358-4. [DOI] [PubMed] [Google Scholar]


