Abstract
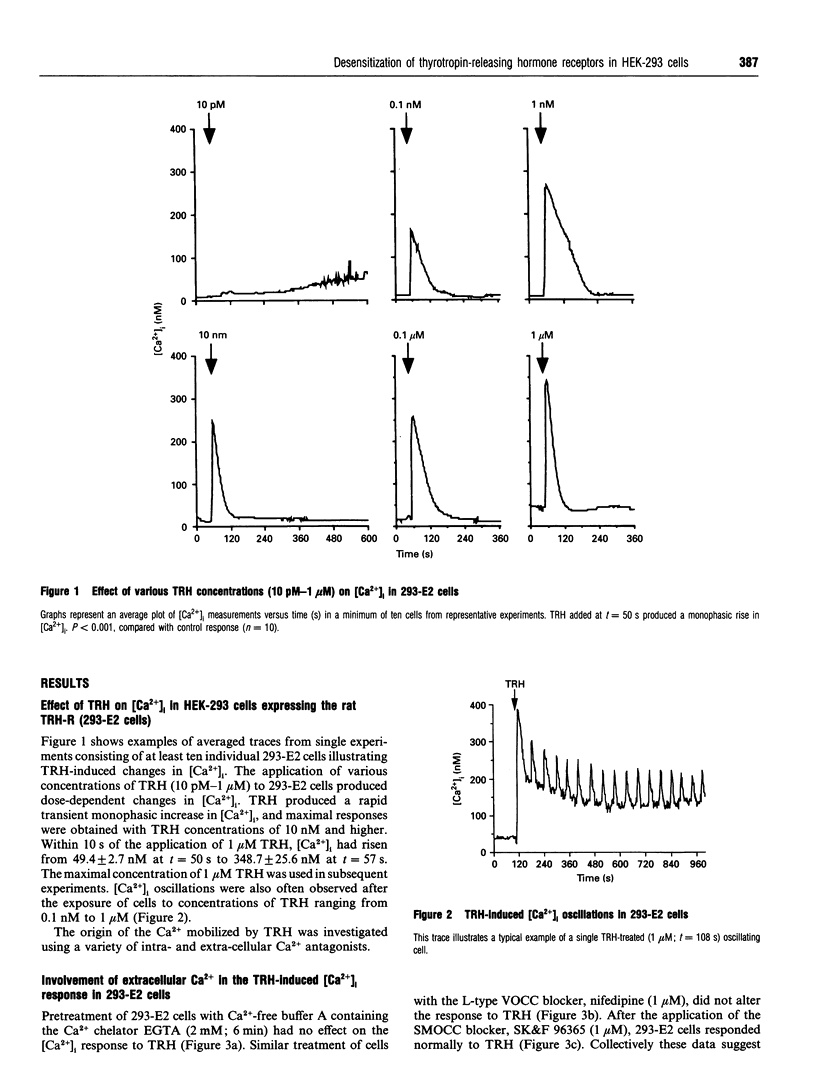
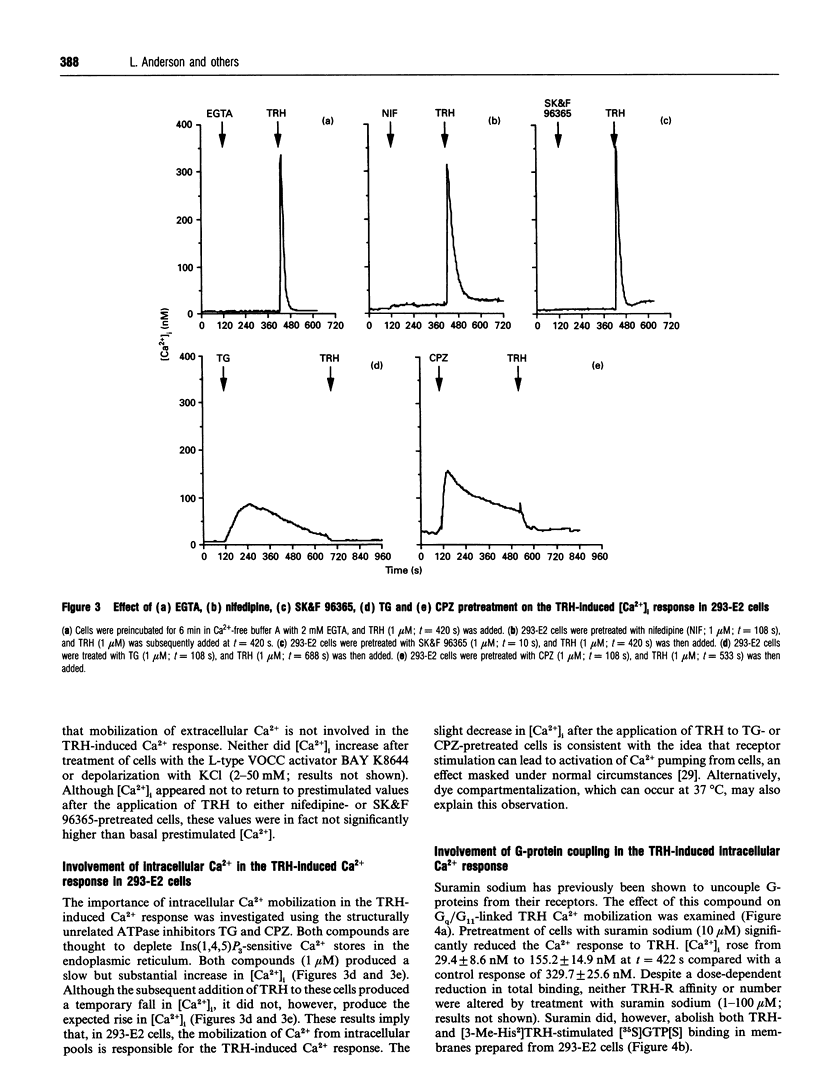
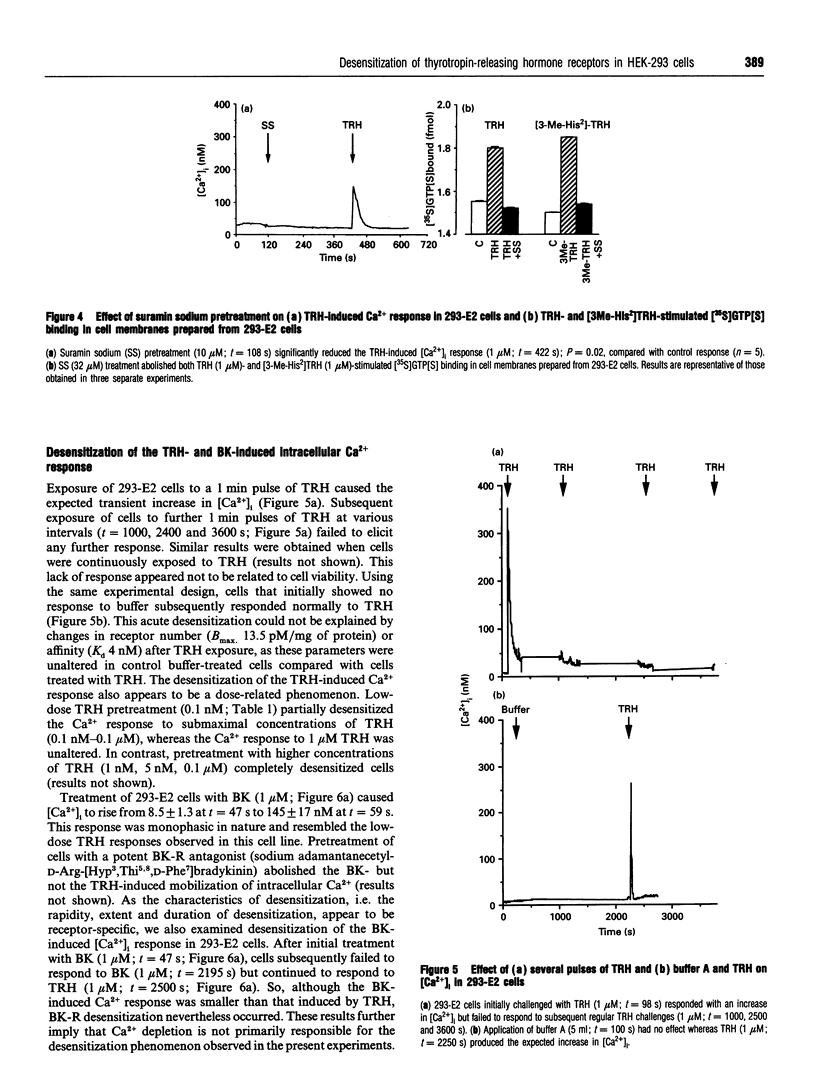
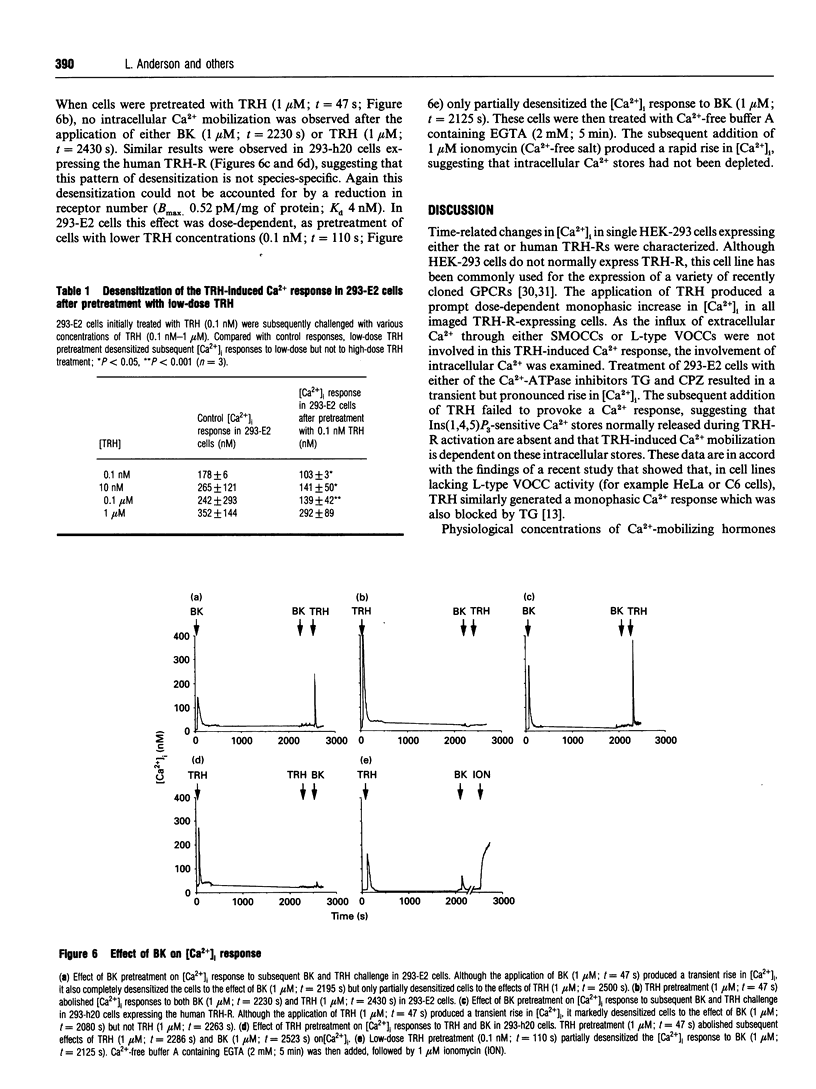
This study uses fluorescence microscopy combined with dynamic video imaging to examine the events associated with the rapid desensitization of the thyrotropin-releasing hormone receptor (TRH-R). In single non-pituitary human embryonic kidney 293 (HEK-293) cells, expressing either the rat or human TRH-Rs, TRH produced a rapid dose-dependent monophasic rise in [Ca2+]i. This Ca2+ transient was completely abolished by pretreatment of cells with the intracellular Ca2+ antagonists thapsigargin or cyclopiazonic acid, but not EGTA, the voltage-operated Ca2+ channel (VOCC) antagonist nifedipine or the second-messenger-operated Ca2+ channel antagonist SK&F 96365. These results suggest that TRH causes the mobilization of Ca2+ from thapsigargin/cyclopiazonic acid-sensitive intracellular Ca2+ stores but not the influx of extracellular Ca2+. HEK-293 cells also failed to respond to KCl or the slow Ca(2+)-channel activator BAY K 8644, suggesting that they lack L-type VOCCs. Rat and human TRH-Rs are highly conserved except at the C-terminus where the sequence differs. The C-terminus is believed to be important in receptor desensitization. Despite differences in this region, rat and human TRH-Rs expressed in HEK-293 cells underwent rapid (within 1 min) desensitization. This desensitization was dose-dependent and did not involve receptor loss. Similarly the bradykinin receptor endogenous to HEK-293 cells also displays a rapid desensitization. We conclude that in TRH-R-expressing non-pituitary HEK-293 cells, TRH mobilizes intracellular Ca2+ resulting in a monophasic Ca2+ transient. The rat and human TRH-Rs as well as the endogenous bradykinin receptor also displayed rapid receptor desensitization.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L., Hoyland J., Mason W. T., Eidne K. A. Characterization of the gonadotrophin-releasing hormone calcium response in single alpha T3-1 pituitary gonadotroph cells. Mol Cell Endocrinol. 1992 Aug;86(3):167–175. doi: 10.1016/0303-7207(92)90141-r. [DOI] [PubMed] [Google Scholar]
- Anderson L., Milligan G., Eidne K. A. Characterization of the gonadotrophin-releasing hormone receptor in alpha T3-1 pituitary gonadotroph cells. J Endocrinol. 1993 Jan;136(1):51–58. doi: 10.1677/joe.0.1360051. [DOI] [PubMed] [Google Scholar]
- Bauer C. K., Davison I., Kubasov I., Schwarz J. R., Mason W. T. Different G proteins are involved in the biphasic response of clonal rat pituitary cells to thyrotropin-releasing hormone. Pflugers Arch. 1994 Aug;428(1):17–25. doi: 10.1007/BF00374747. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Calcium oscillations. J Biol Chem. 1990 Jun 15;265(17):9583–9586. [PubMed] [Google Scholar]
- Bird G. S., Rossier M. F., Hughes A. R., Shears S. B., Armstrong D. L., Putney J. W., Jr Activation of Ca2+ entry into acinar cells by a non-phosphorylatable inositol trisphosphate. Nature. 1991 Jul 11;352(6331):162–165. doi: 10.1038/352162a0. [DOI] [PubMed] [Google Scholar]
- Butler S. J., Kelly E. C., McKenzie F. R., Guild S. B., Wakelam M. J., Milligan G. Differential effects of suramin on the coupling of receptors to individual species of pertussis-toxin-sensitive guanine-nucleotide-binding proteins. Biochem J. 1988 Apr 1;251(1):201–205. doi: 10.1042/bj2510201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiavaroli C., Vacher P., Schlegel W. Modulation of Ca2+ influx by protein phosphorylation in single intact clonal pituitary cells. Eur J Pharmacol. 1992 Oct 1;227(2):173–180. doi: 10.1016/0922-4106(92)90125-f. [DOI] [PubMed] [Google Scholar]
- Cubitt A. B., Geras-Raaka E., Gershengorn M. C. Thyrotropin-releasing hormone receptor occupancy determines the fraction of the responsive pool of inositol lipids hydrolysed in rat pituitary tumour cells. Biochem J. 1990 Oct 15;271(2):331–336. doi: 10.1042/bj2710331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasso L. L., Taylor C. W. Heparin and other polyanions uncouple alpha 1-adrenoceptors from G-proteins. Biochem J. 1991 Dec 15;280(Pt 3):791–795. doi: 10.1042/bj2800791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond A. H., Bushfield M., Macphee C. H. Thyrotropin-releasing hormone-stimulated [3H]inositol metabolism in GH3 pituitary tumor cells. Studies with lithium. Mol Pharmacol. 1984 Mar;25(2):201–208. [PubMed] [Google Scholar]
- Duthie S. M., Taylor P. L., Anderson L., Cook J., Eidne K. A. Cloning and functional characterisation of the human TRH receptor. Mol Cell Endocrinol. 1993 Sep;95(1-2):R11–R15. doi: 10.1016/0303-7207(93)90043-j. [DOI] [PubMed] [Google Scholar]
- Falck-Pedersen E., Heinflink M., Alvira M., Nussenzveig D. R., Gershengorn M. C. Expression of thyrotropin-releasing hormone receptors by adenovirus-mediated gene transfer reveals that thyrotropin-releasing hormone desensitization is cell specific. Mol Pharmacol. 1994 Apr;45(4):684–689. [PubMed] [Google Scholar]
- Fujimoto J., Narayanan C. S., Benjamin J. E., Gershengorn M. C. Posttranscriptional up-regulation of thyrotropin-releasing hormone (TRH) receptor messenger ribonucleic acid by TRH in COS-1 cells transfected with mouse pituitary TRH receptor complementary deoxyribonucleic acid. Endocrinology. 1992 Oct;131(4):1716–1720. doi: 10.1210/endo.131.4.1327718. [DOI] [PubMed] [Google Scholar]
- Hausdorff W. P., Bouvier M., O'Dowd B. F., Irons G. P., Caron M. G., Lefkowitz R. J. Phosphorylation sites on two domains of the beta 2-adrenergic receptor are involved in distinct pathways of receptor desensitization. J Biol Chem. 1989 Jul 25;264(21):12657–12665. [PubMed] [Google Scholar]
- Hawes B. E., Conn P. M. Development of gonadotrope desensitization to gonadotropin-releasing hormone (GnRH) and recovery are not coupled to inositol phosphate production or GnRH receptor number. Endocrinology. 1992 Dec;131(6):2681–2689. doi: 10.1210/endo.131.6.1332845. [DOI] [PubMed] [Google Scholar]
- Hsieh K. P., Martin T. F. Thyrotropin-releasing hormone and gonadotropin-releasing hormone receptors activate phospholipase C by coupling to the guanosine triphosphate-binding proteins Gq and G11. Mol Endocrinol. 1992 Oct;6(10):1673–1681. doi: 10.1210/mend.6.10.1333052. [DOI] [PubMed] [Google Scholar]
- Huang R. R., Dehaven R. N., Cheung A. H., Diehl R. E., Dixon R. A., Strader C. D. Identification of allosteric antagonists of receptor-guanine nucleotide-binding protein interactions. Mol Pharmacol. 1990 Feb;37(2):304–310. [PubMed] [Google Scholar]
- Imai A., Gershengorn M. C. Evidence for tight coupling of thyrotropin-releasing hormone receptors to stimulated inositol trisphosphate formation in rat pituitary cells. J Biol Chem. 1985 Sep 5;260(19):10536–10540. [PubMed] [Google Scholar]
- Kim G. D., Carr I. C., Anderson L. A., Zabavnik J., Eidne K. A., Milligan G. The long isoform of the rat thyrotropin-releasing hormone receptor down-regulates Gq proteins. J Biol Chem. 1994 Aug 5;269(31):19933–19940. [PubMed] [Google Scholar]
- Klueppelberg U. G., Gates L. K., Gorelick F. S., Miller L. J. Agonist-regulated phosphorylation of the pancreatic cholecystokinin receptor. J Biol Chem. 1991 Feb 5;266(4):2403–2408. [PubMed] [Google Scholar]
- Kobilka B. Adrenergic receptors as models for G protein-coupled receptors. Annu Rev Neurosci. 1992;15:87–114. doi: 10.1146/annurev.ne.15.030192.000511. [DOI] [PubMed] [Google Scholar]
- Kopp R., Pfeiffer A. Suramin alters phosphoinositide synthesis and inhibits growth factor receptor binding in HT-29 cells. Cancer Res. 1990 Oct 15;50(20):6490–6496. [PubMed] [Google Scholar]
- Kwatra M. M., Schwinn D. A., Schreurs J., Blank J. L., Kim C. M., Benovic J. L., Krause J. E., Caron M. G., Lefkowitz R. J. The substance P receptor, which couples to Gq/11, is a substrate of beta-adrenergic receptor kinase 1 and 2. J Biol Chem. 1993 May 5;268(13):9161–9164. [PubMed] [Google Scholar]
- Lakkakorpi J. T., Rajaniemi H. J. Regulation of intracellular free Ca2+ by the LH/CG receptor in an established cell line 293 expressing transfected rat receptor. Mol Cell Endocrinol. 1994 Feb;99(1):39–47. doi: 10.1016/0303-7207(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Law G. J., Pachter J. A., Dannies P. S. Ca2+ transients induced by thyrotropin-releasing hormone rapidly lose their ability to cause release of prolactin. Mol Endocrinol. 1989 Mar;3(3):539–546. doi: 10.1210/mend-3-3-539. [DOI] [PubMed] [Google Scholar]
- Li P., Thaw C. N., Sempowski G. D., Gershengorn M. C., Hinkle P. M. Characterization of the calcium response to thyrotropin-releasing hormone (TRH) in cells transfected with TRH receptor complementary DNA: importance of voltage-sensitive calcium channels. Mol Endocrinol. 1992 Sep;6(9):1393–1402. doi: 10.1210/mend.6.9.1279382. [DOI] [PubMed] [Google Scholar]
- Lorenzen A., Fuss M., Vogt H., Schwabe U. Measurement of guanine nucleotide-binding protein activation by A1 adenosine receptor agonists in bovine brain membranes: stimulation of guanosine-5'-O-(3-[35S]thio)triphosphate binding. Mol Pharmacol. 1993 Jul;44(1):115–123. [PubMed] [Google Scholar]
- Menniti F. S., Takemura H., Oliver K. G., Putney J. W., Jr Different modes of regulation for receptors activating phospholipase C in the rat pancreatoma cell line AR4-2J. Mol Pharmacol. 1991 Nov;40(5):727–733. [PubMed] [Google Scholar]
- Nagayama Y., Chazenbalk G. D., Takeshita A., Kimura H., Ashizawa K., Yokoyama N., Rapoport B., Nagataki S. Studies on homologous desensitization of the thyrotropin receptor in 293 human embryonal kidney cells. Endocrinology. 1994 Sep;135(3):1060–1065. doi: 10.1210/endo.135.3.8070347. [DOI] [PubMed] [Google Scholar]
- Nelson E. J., Hinkle P. M. Characteristics of the Ca2+ spike and oscillations induced by different doses of thyrotropin-releasing hormone (TRH) in individual pituitary cells and nonexcitable cells transfected with TRH receptor complementary deoxyribonucleic acid. Endocrinology. 1994 Sep;135(3):1084–1092. doi: 10.1210/endo.135.3.8070350. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992 Oct 23;258(5082):607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Nussenzveig D. R., Heinflink M., Gershengorn M. C. Agonist-stimulated internalization of the thyrotropin-releasing hormone receptor is dependent on two domains in the receptor carboxyl terminus. J Biol Chem. 1993 Feb 5;268(4):2389–2392. [PubMed] [Google Scholar]
- Perlman J. H., Gershengorn M. C. Thyrotropin-releasing hormone stimulation of phosphoinositide hydrolysis desensitizes. Evidence against mediation by protein kinase C or calcium. Endocrinology. 1991 Nov;129(5):2679–2686. doi: 10.1210/endo-129-5-2679. [DOI] [PubMed] [Google Scholar]
- Probst W. C., Snyder L. A., Schuster D. I., Brosius J., Sealfon S. C. Sequence alignment of the G-protein coupled receptor superfamily. DNA Cell Biol. 1992 Jan-Feb;11(1):1–20. doi: 10.1089/dna.1992.11.1. [DOI] [PubMed] [Google Scholar]
- Richardson R. M., Kim C., Benovic J. L., Hosey M. M. Phosphorylation and desensitization of human m2 muscarinic cholinergic receptors by two isoforms of the beta-adrenergic receptor kinase. J Biol Chem. 1993 Jun 25;268(18):13650–13656. [PubMed] [Google Scholar]
- Sellar R. E., Taylor P. L., Lamb R. F., Zabavnik J., Anderson L., Eidne K. A. Functional expression and molecular characterization of the thyrotrophin-releasing hormone receptor from the rat anterior pituitary gland. J Mol Endocrinol. 1993 Apr;10(2):199–206. doi: 10.1677/jme.0.0100199. [DOI] [PubMed] [Google Scholar]
- Shorte S. L., Schofield J. G. Thyrotropin-releasing hormone-induced cytosolic calcium transients: characterisation of store refilling in bovine anterior pituitary cells. Mol Cell Endocrinol. 1991 Aug;79(1-3):167–176. doi: 10.1016/0303-7207(91)90107-4. [DOI] [PubMed] [Google Scholar]
- Stauderman K. A., Pruss R. M. Different patterns of agonist-stimulated increases of 3H-inositol phosphate isomers and cytosolic Ca2+ in bovine adrenal chromaffin cells: comparison of the effects of histamine and angiotensin II. J Neurochem. 1990 Mar;54(3):946–953. doi: 10.1111/j.1471-4159.1990.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Stauderman K. A., Pruss R. M. Different patterns of agonist-stimulated increases of 3H-inositol phosphate isomers and cytosolic Ca2+ in bovine adrenal chromaffin cells: comparison of the effects of histamine and angiotensin II. J Neurochem. 1990 Mar;54(3):946–953. doi: 10.1111/j.1471-4159.1990.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Straub R. E., Frech G. C., Joho R. H., Gershengorn M. C. Expression cloning of a cDNA encoding the mouse pituitary thyrotropin-releasing hormone receptor. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9514–9518. doi: 10.1073/pnas.87.24.9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin A. B., Nahorski S. R. Rapid agonist-mediated phosphorylation of m3-muscarinic receptors revealed by immunoprecipitation. J Biol Chem. 1993 May 5;268(13):9817–9823. [PubMed] [Google Scholar]
- Torjesen P. A., Bjøro T., Ostberg B. C., Haug E. Thyrotropin-releasing hormone-stimulated inositol trisphosphate formation is liable to thyrotropin-releasing hormone-induced desensitization by a calcium-dependent mechanism. Mol Cell Endocrinol. 1988 Mar;56(1-2):107–114. doi: 10.1016/0303-7207(88)90014-7. [DOI] [PubMed] [Google Scholar]
- Van Zoelen E. J., Peters P. H., Afink G. B., Van Genesen S., De Roos D. G., Van Rotterdam W., Theuvenet A. P. Bradykinin-induced growth inhibition of normal rat kidney (NRK) cells is paralleled by a decrease in epidermal-growth-factor receptor expression. Biochem J. 1994 Mar 1;298(Pt 2):335–340. doi: 10.1042/bj2980335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner K. A., Yacono P. W., Golan D. E., Tashjian A. H., Jr Mechanism of spontaneous intracellular calcium fluctuations in single GH4C1 rat pituitary cells. Biochem J. 1993 May 15;292(Pt 1):175–182. doi: 10.1042/bj2920175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winicov I., Gershengorn M. C. Receptor density determines secretory response patterns mediate by inositol lipid-derived second messengers. Comparison of thyrotropin-releasing hormone and carbamylcholine actions in thyroid-stimulating hormone-secreting mouse pituitary tumor cells. J Biol Chem. 1989 Jun 5;264(16):9438–9443. [PubMed] [Google Scholar]
- Winiger B. P., Schlegel W. Rapid transient elevations of cytosolic calcium triggered by thyrotropin releasing hormone in individual cells of the pituitary line GH3B6. Biochem J. 1988 Oct 1;255(1):161–167. doi: 10.1042/bj2550161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M., Monden T., Satoh T., Iizuka M., Murakami M., Iriuchijima T., Mori M. Differential regulation of thyrotropin-releasing hormone receptor mRNA levels by thyroid hormone in vivo and in vitro (GH3 cells). Biochem Biophys Res Commun. 1992 Apr 15;184(1):367–372. doi: 10.1016/0006-291x(92)91202-2. [DOI] [PubMed] [Google Scholar]
- Zhang B. X., Zhao H., Loessberg P., Muallem S. Activation of the plasma membrane Ca2+ pump during agonist stimulation of pancreatic acini. J Biol Chem. 1992 Aug 5;267(22):15419–15425. [PubMed] [Google Scholar]


