Abstract
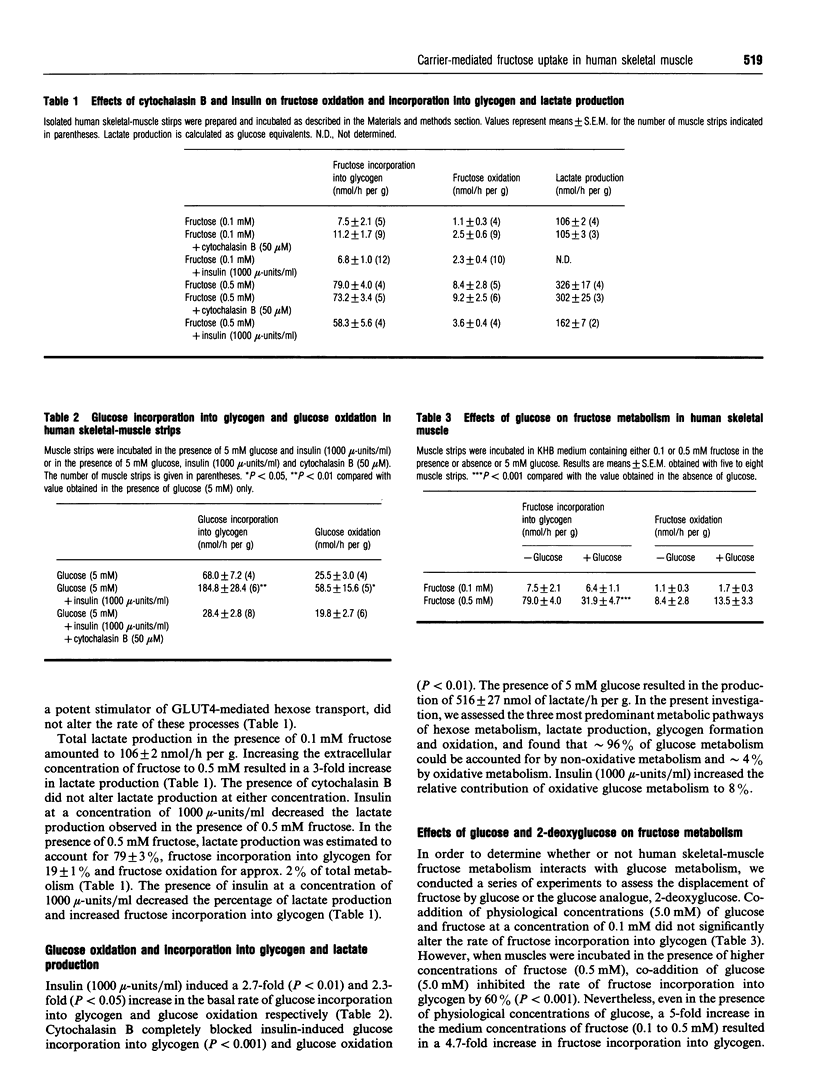
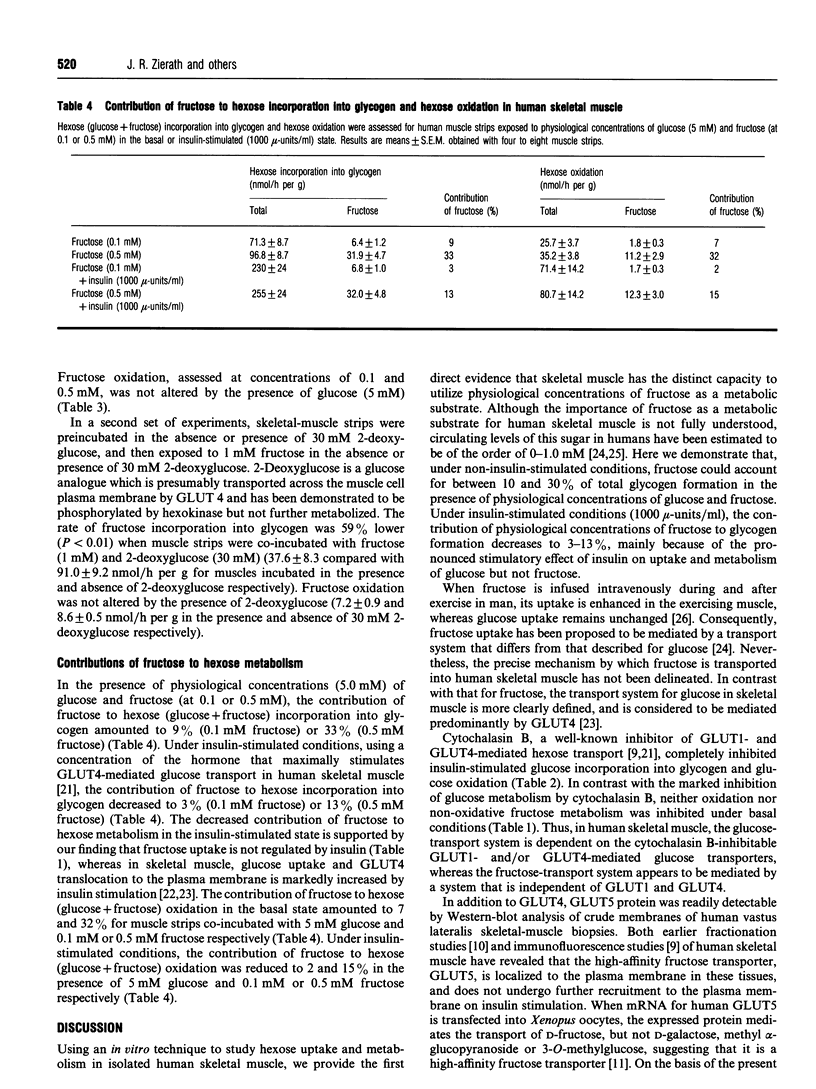
To determine whether fructose can be utilized as a metabolic substrate for skeletal muscle in man, we investigated its incorporation into glycogen, its oxidation and lactate production in isolated human skeletal muscle. Rates of fructose oxidation and incorporation into glycogen increased in the presence of increasing fructose concentrations (0.1-1.0 mM). Lactate production increased 3-fold when extracellular fructose was increased from 0.1 to 0.5 mM. Cytochalasin B, a competitive inhibitor of hexose transport mediated by the GLUT1 and GLUT4 facilitative glucose transporters, completely inhibited insulin-stimulated glucose incorporation into glycogen and glucose oxidation (P < 0.01), but did not alter fructose incorporation into glycogen or fructose oxidation. Insulin (1000 mu-units/ml) increased glucose incorporation into glycogen 2.7-fold and glucose oxidation 2.3-fold, whereas no effect on fructose incorporation into glycogen or fructose oxidation was noted. A physiological concentration of glucose (5 mM) decreased the rate of 0.5 mM fructose incorporation into glycogen by 60% (P < 0.001), whereas fructose oxidation was not altered in the presence of 5 mM glucose. Irrespective of fructose concentration, the majority of fructose taken up underwent non-oxidative metabolism. Lactate production accounted for approx. 80% of the fructose metabolism in the basal state and approx. 70% in the insulin (1000 mu-units/ml)-stimulated state. In the presence of 5 mM glucose, physiological concentrations of fructose could account for approximately 10-30% of hexose (glucose + fructose) incorporation into glycogen under non-insulin-stimulated conditions. In conclusion, fructose appears to be transported into human skeletal muscle via a carrier-mediated system that does not involve GLUT4 or GLUT1. Furthermore, under physiological conditions, fructose can significantly contribute to carbohydrate metabolism in human skeletal muscle.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlborg G., Björkman O. Splanchnic and muscle fructose metabolism during and after exercise. J Appl Physiol (1985) 1990 Oct;69(4):1244–1251. doi: 10.1152/jappl.1990.69.4.1244. [DOI] [PubMed] [Google Scholar]
- Bantle J. P. Clinical aspects of sucrose and fructose metabolism. Diabetes Care. 1989 Jan;12(1):56–82. doi: 10.2337/diacare.12.1.56. [DOI] [PubMed] [Google Scholar]
- Bergström J., Hultman E. Synthesis of muscle glycogen in man after glucose and fructose infusion. Acta Med Scand. 1967 Jul;182(1):93–107. doi: 10.1111/j.0954-6820.1967.tb11503.x. [DOI] [PubMed] [Google Scholar]
- Burant C. F., Takeda J., Brot-Laroche E., Bell G. I., Davidson N. O. Fructose transporter in human spermatozoa and small intestine is GLUT5. J Biol Chem. 1992 Jul 25;267(21):14523–14526. [PubMed] [Google Scholar]
- Cuendet G. S., Loten E. G., Jeanrenaud B., Renold A. E. Decreased basal, noninsulin-stimulated glucose uptake and metabolism by skeletal soleus muscle isolated from obese-hyperglycemic (ob/ob) mice. J Clin Invest. 1976 Nov;58(5):1078–1088. doi: 10.1172/JCI108559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delarue J., Normand S., Pachiaudi C., Beylot M., Lamisse F., Riou J. P. The contribution of naturally labelled 13C fructose to glucose appearance in humans. Diabetologia. 1993 Apr;36(4):338–345. doi: 10.1007/BF00400238. [DOI] [PubMed] [Google Scholar]
- Friedman J. E., Caro J. F., Pories W. J., Azevedo J. L., Jr, Dohm G. L. Glucose metabolism in incubated human muscle: effect of obesity and non-insulin-dependent diabetes mellitus. Metabolism. 1994 Aug;43(8):1047–1054. doi: 10.1016/0026-0495(94)90188-0. [DOI] [PubMed] [Google Scholar]
- Gumà A., Zierath J. R., Wallberg-Henriksson H., Klip A. Insulin induces translocation of GLUT-4 glucose transporters in human skeletal muscle. Am J Physiol. 1995 Apr;268(4 Pt 1):E613–E622. doi: 10.1152/ajpendo.1995.268.4.E613. [DOI] [PubMed] [Google Scholar]
- HOLDSWORTH C. D., DAWSON A. M. ABSORPTION OF FRUCTOSE IN MAN. Proc Soc Exp Biol Med. 1965 Jan;118:142–145. doi: 10.3181/00379727-118-29780. [DOI] [PubMed] [Google Scholar]
- Hermansen L., Vaage O. Lactate disappearance and glycogen synthesis in human muscle after maximal exercise. Am J Physiol. 1977 Nov;233(5):E422–E429. doi: 10.1152/ajpendo.1977.233.5.E422. [DOI] [PubMed] [Google Scholar]
- Hundal H. S., Ahmed A., Gumà A., Mitsumoto Y., Marette A., Rennie M. J., Klip A. Biochemical and immunocytochemical localization of the 'GLUT5 glucose transporter' in human skeletal muscle. Biochem J. 1992 Sep 1;286(Pt 2):339–343. doi: 10.1042/bj2860339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund S., Holman G. D., Schmitz O., Pedersen O. Glut 4 content in the plasma membrane of rat skeletal muscle: comparative studies of the subcellular fractionation method and the exofacial photolabelling technique using ATB-BMPA. FEBS Lett. 1993 Sep 20;330(3):312–318. doi: 10.1016/0014-5793(93)80895-2. [DOI] [PubMed] [Google Scholar]
- Mayes P. A. Intermediary metabolism of fructose. Am J Clin Nutr. 1993 Nov;58(5 Suppl):754S–765S. doi: 10.1093/ajcn/58.5.754S. [DOI] [PubMed] [Google Scholar]
- Nilsson L. H., Hultman E. Liver and muscle glycogen in man after glucose and fructose infusion. Scand J Clin Lab Invest. 1974 Feb;33(1):5–10. doi: 10.3109/00365517409114190. [DOI] [PubMed] [Google Scholar]
- Park Y. K., Yetley E. A. Intakes and food sources of fructose in the United States. Am J Clin Nutr. 1993 Nov;58(5 Suppl):737S–747S. doi: 10.1093/ajcn/58.5.737S. [DOI] [PubMed] [Google Scholar]
- Shepherd P. R., Gibbs E. M., Wesslau C., Gould G. W., Kahn B. B. Human small intestine facilitative fructose/glucose transporter (GLUT5) is also present in insulin-responsive tissues and brain. Investigation of biochemical characteristics and translocation. Diabetes. 1992 Oct;41(10):1360–1365. doi: 10.2337/diab.41.10.1360. [DOI] [PubMed] [Google Scholar]
- Stauffacher W., Renold A. E. Effect of insulin in vivo on diaphragm and adipose tissue of obese mice. Am J Physiol. 1969 Jan;216(1):98–105. doi: 10.1152/ajplegacy.1969.216.1.98. [DOI] [PubMed] [Google Scholar]
- Vinten J. Cytochalasin B inhibition and temperature dependence of 3-O-methylglucose transport in fat cells. Biochim Biophys Acta. 1978 Aug 4;511(2):259–273. doi: 10.1016/0005-2736(78)90319-x. [DOI] [PubMed] [Google Scholar]
- WEICHSELBAUM T. E., MARGRAF H. W., ELMAN R. Metabolism of intravenously infused fructose in man. Metabolism. 1953 Sep;2(5):434–449. [PubMed] [Google Scholar]
- Wallberg-Henriksson H. Glucose transport into skeletal muscle. Influence of contractile activity, insulin, catecholamines and diabetes mellitus. Acta Physiol Scand Suppl. 1987;564:1–80. [PubMed] [Google Scholar]
- Wallberg-Henriksson H., Zetan N., Henriksson J. Reversibility of decreased insulin-stimulated glucose transport capacity in diabetic muscle with in vitro incubation. Insulin is not required. J Biol Chem. 1987 Jun 5;262(16):7665–7671. [PubMed] [Google Scholar]
- Young D. A., Ho R. S., Bell P. A., Cohen D. K., McIntosh R. H., Nadelson J., Foley J. E. Inhibition of hepatic glucose production by SDZ 51641. Diabetes. 1990 Nov;39(11):1408–1413. doi: 10.2337/diab.39.11.1408. [DOI] [PubMed] [Google Scholar]
- Zierath J. R., Galuska D., Engström A., Johnson K. H., Betsholtz C., Westermark P., Wallberg-Henriksson H. Human islet amyloid polypeptide at pharmacological levels inhibits insulin and phorbol ester-stimulated glucose transport in in vitro incubated human muscle strips. Diabetologia. 1992 Jan;35(1):26–31. doi: 10.1007/BF00400848. [DOI] [PubMed] [Google Scholar]
- Zierath J. R., Galuska D., Nolte L. A., Thörne A., Kristensen J. S., Wallberg-Henriksson H. Effects of glycaemia on glucose transport in isolated skeletal muscle from patients with NIDDM: in vitro reversal of muscular insulin resistance. Diabetologia. 1994 Mar;37(3):270–277. doi: 10.1007/BF00398054. [DOI] [PubMed] [Google Scholar]


