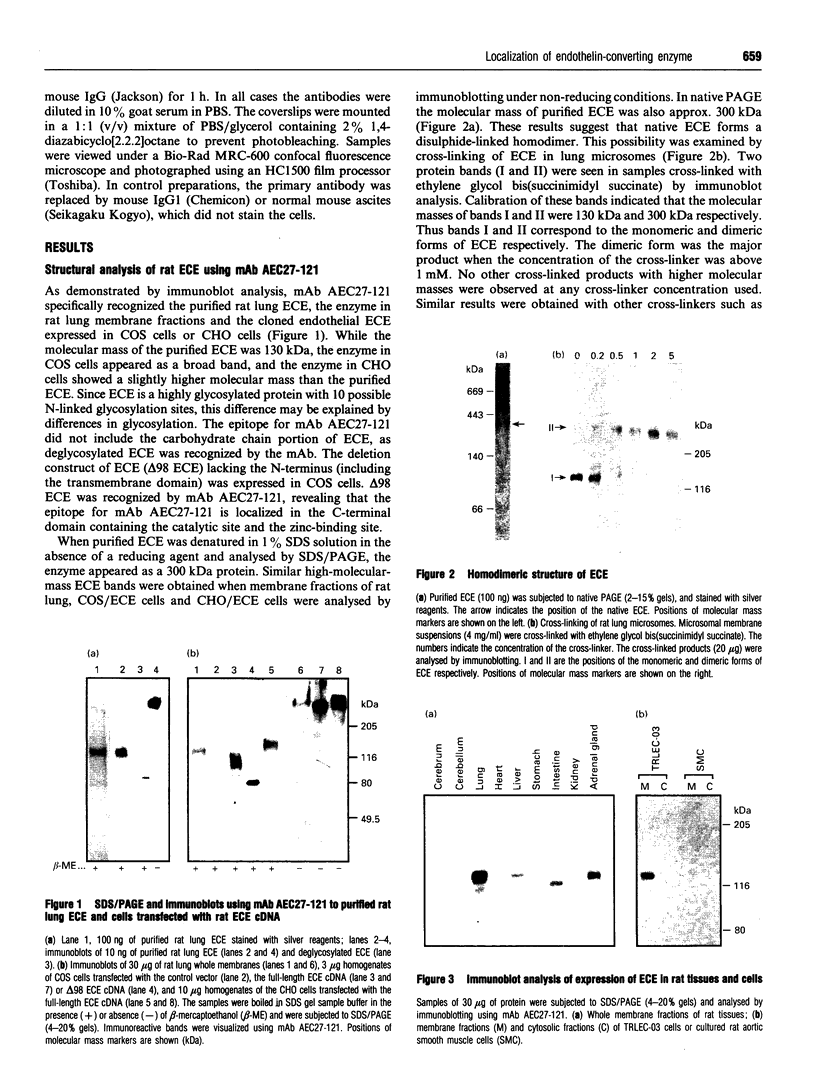
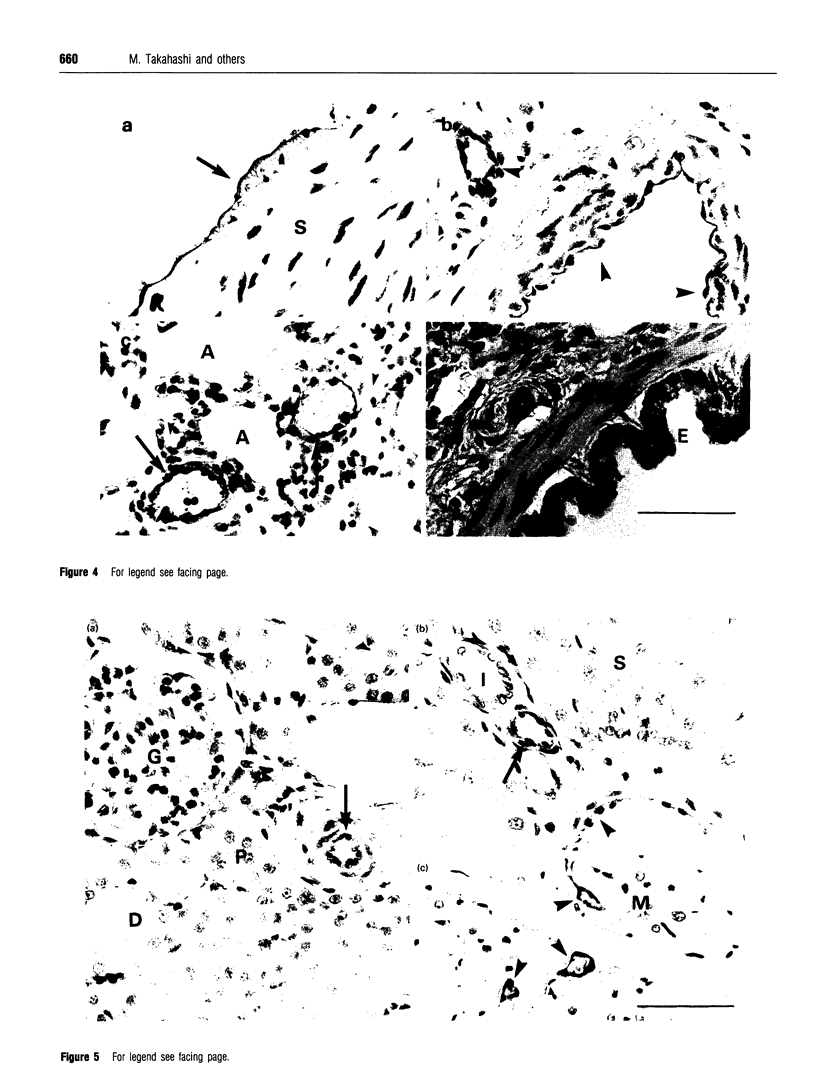
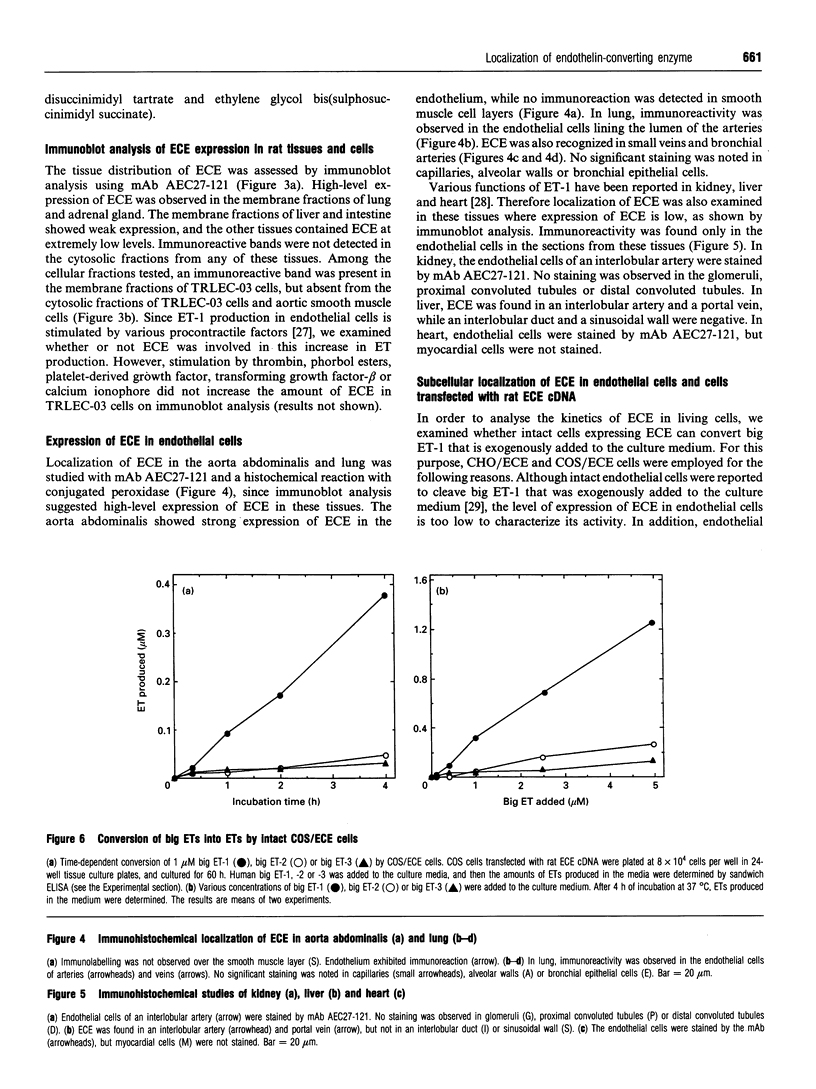
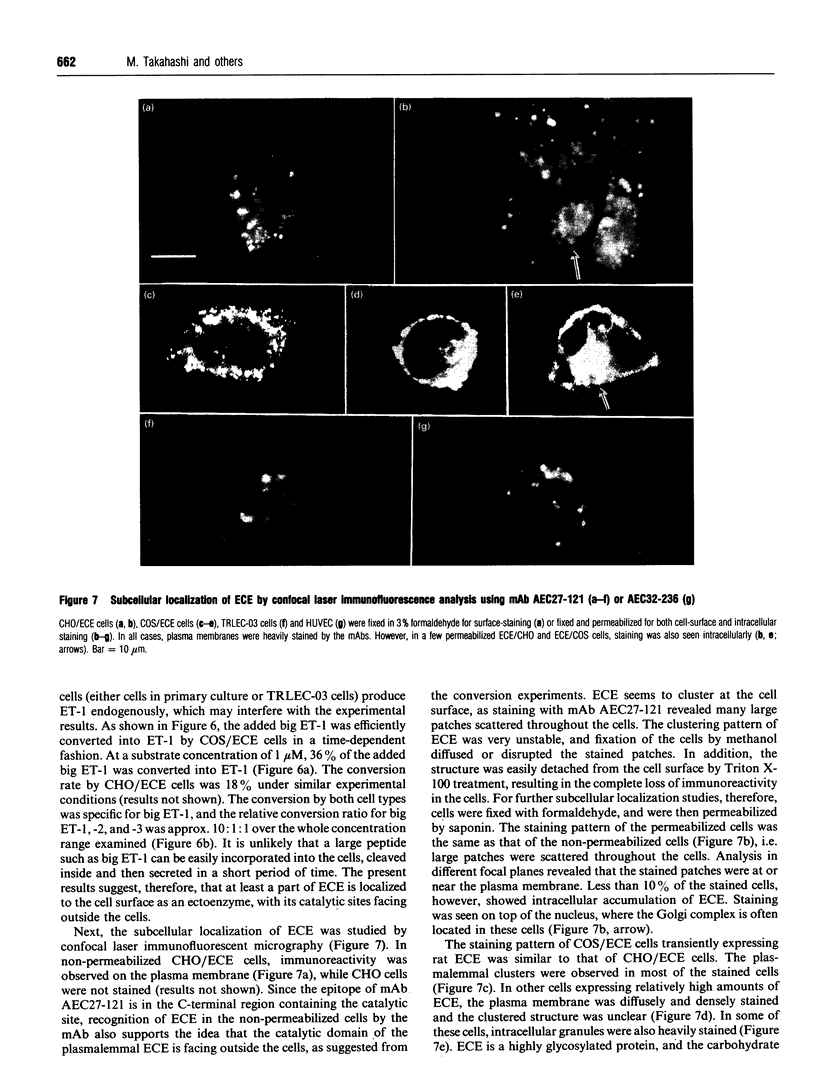
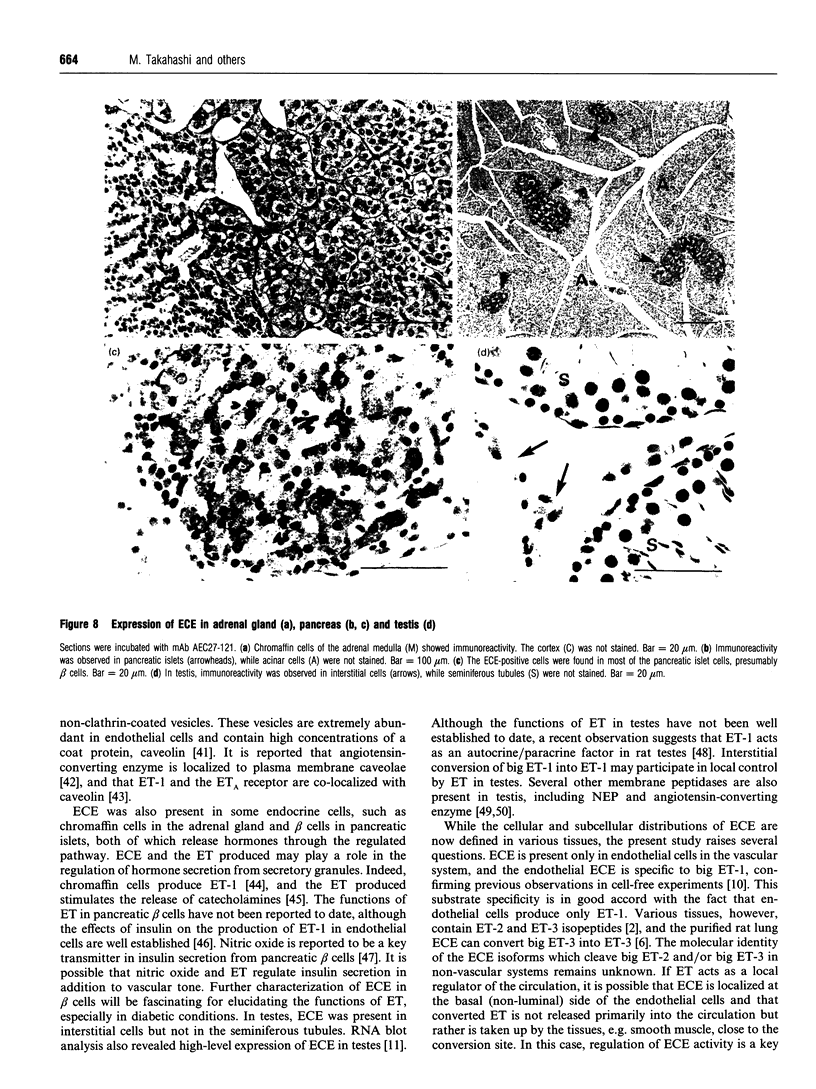
Abstract
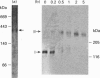
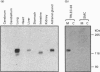
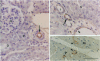
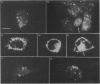
Endothelin is a potent vasoconstrictive peptide that is produced by vascular endothelial cells; it is formed from its precursor, big endothelin, by endothelin-converting enzyme (ECE). In this work, ECE was studied using specific monoclonal antibodies. In immunoblotting, ECE was estimated to be a 300 kDa protein on SDS/PAGE under non-reducing conditions, and 130 kDa under reducing conditions. Cross-linking experiments revealed that ECE is composed of two disulphide-linked subunits. Localization of ECE was studied at the cellular and subcellular levels in various rat tissues and cells. High-level expression of ECE was observed in membrane fractions of simian virus 40-transformed rat endothelial cells by immunoblotting, but the immunoreactive band was absent form aortic smooth muscle cells and cytosolic fractions of endothelial cells. In immunohistochemical analysis, ECE was found to be localized in the endothelial cells of the aorta, lung, kidney, liver and heart. Confocal immunofluorescent microscopy showed that most of the ECE in endothelial cells and cells transfected with ECE cDNA was clustered along the plasma membrane. Intact COS or CHO cells transfected with ECE cDNA rapidly and efficiently cleaved big endothelin-1 added to the culture medium. Thus endothelial cells express ECE on the plasma membrane and the active site of the enzyme faces outside the cells, i.e. it is an ectoenzyme. Other than endothelial cells, ECE was also present in some secretory cells. The enzyme was abundant in the adrenal gland, and localized in chromaffin cells. ECE was also highly condensed in pancreatic islet beta cells. It is concluded that ECE and endothelin may be involved in the regulated secretion of hormones.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahn K., Beningo K., Olds G., Hupe D. The endothelin-converting enzyme from human umbilical vein is a membrane-bound metalloprotease similar to that from bovine aortic endothelial cells. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8606–8610. doi: 10.1073/pnas.89.18.8606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boarder M. R., Marriott D. B. Characterization of endothelin-1 stimulation of catecholamine release from adrenal chromaffin cells. J Cardiovasc Pharmacol. 1989;13 (Suppl 5):S223–S224. doi: 10.1097/00005344-198900135-00066. [DOI] [PubMed] [Google Scholar]
- Chamley-Campbell J. H., Campbell G. R., Ross R. Phenotype-dependent response of cultured aortic smooth muscle to serum mitogens. J Cell Biol. 1981 May;89(2):379–383. doi: 10.1083/jcb.89.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun M., Liyanage U. K., Lisanti M. P., Lodish H. F. Signal transduction of a G protein-coupled receptor in caveolae: colocalization of endothelin and its receptor with caveolin. Proc Natl Acad Sci U S A. 1994 Nov 22;91(24):11728–11732. doi: 10.1073/pnas.91.24.11728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Devault A., Lazure C., Nault C., Le Moual H., Seidah N. G., Chrétien M., Kahn P., Powell J., Mallet J., Beaumont A. Amino acid sequence of rabbit kidney neutral endopeptidase 24.11 (enkephalinase) deduced from a complementary DNA. EMBO J. 1987 May;6(5):1317–1322. doi: 10.1002/j.1460-2075.1987.tb02370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty A. M. Endothelin: a new challenge. J Med Chem. 1992 May 1;35(9):1493–1508. doi: 10.1021/jm00087a001. [DOI] [PubMed] [Google Scholar]
- Emori T., Hirata Y., Ohta K., Shichiri M., Shimokado K., Marumo F. Concomitant secretion of big endothelin and its C-terminal fragment from human and bovine endothelial cells. Biochem Biophys Res Commun. 1989 Jul 14;162(1):217–223. doi: 10.1016/0006-291x(89)91984-0. [DOI] [PubMed] [Google Scholar]
- Erdös E. G., Schulz W. W., Gafford J. T., Defendini R. Neutral metalloendopeptidase in human male genital tract. Comparison to angiotensin I-converting enzyme. Lab Invest. 1985 Apr;52(4):437–447. [PubMed] [Google Scholar]
- Fantoni G., Morris P. L., Forti G., Vannelli G. B., Orlando C., Barni T., Sestini R., Danza G., Maggi M. Endothelin-1: a new autocrine/paracrine factor in rat testis. Am J Physiol. 1993 Aug;265(2 Pt 1):E267–E274. doi: 10.1152/ajpendo.1993.265.2.E267. [DOI] [PubMed] [Google Scholar]
- Fulcher I. S., Kenny A. J. Proteins of the kidney microvillar membrane. The amphipathic forms of endopeptidase purified from pig kidneys. Biochem J. 1983 Jun 1;211(3):743–753. doi: 10.1042/bj2110743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee N. S., Matsas R., Kenny A. J. A monoclonal antibody to kidney endopeptidase-24.11. Its application in immunoadsorbent purification of the enzyme and immunofluorescent microscopy of kidney and intestine. Biochem J. 1983 Aug 15;214(2):377–386. doi: 10.1042/bj2140377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hioki Y., Okada K., Ito H., Matsuyama K., Yano M. Endothelin converting enzyme of bovine carotid artery smooth muscles. Biochem Biophys Res Commun. 1991 Jan 31;174(2):446–451. doi: 10.1016/0006-291x(91)91436-g. [DOI] [PubMed] [Google Scholar]
- Hoch W., Campanelli J. T., Scheller R. H. Agrin-induced clustering of acetylcholine receptors: a cytoskeletal link. J Cell Biol. 1994 Jul;126(1):1–4. doi: 10.1083/jcb.126.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikura T., Sawamura T., Shiraki T., Hosokawa H., Kido T., Hoshikawa H., Shimada K., Tanzawa K., Kobayashi S., Miwa S. cDNA cloning and expression of bovine endothelin converting enzyme. Biochem Biophys Res Commun. 1994 Sep 30;203(3):1417–1422. doi: 10.1006/bbrc.1994.2343. [DOI] [PubMed] [Google Scholar]
- Inoue A., Yanagisawa M., Kimura S., Kasuya Y., Miyauchi T., Goto K., Masaki T. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2863–2867. doi: 10.1073/pnas.86.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaw S., Hecker M., Vane J. R. The two-step conversion of big endothelin 1 to endothelin 1 and degradation of endothelin 1 by subcellular fractions from human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6886–6890. doi: 10.1073/pnas.89.15.6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S., Kasuya Y., Sawamura T., Shinimi O., Sugita Y., Yanagisawa M., Goto K., Masaki T. Conversion of big endothelin-1 to 21-residue endothelin-1 is essential for expression of full vasoconstrictor activity: structure-activity relationships of big endothelin-1. J Cardiovasc Pharmacol. 1989;13 (Suppl 5):S5–S18. doi: 10.1097/00005344-198900135-00003. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Littlewood G. M., Hooper N. M., Turner A. J. Ectoenzymes of the kidney microvillar membrane. Affinity purification, characterization and localization of the phospholipase C-solubilized form of renal dipeptidase. Biochem J. 1989 Jan 15;257(2):361–367. doi: 10.1042/bj2570361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand P., Tang J., Bond J. S. Membrane association and oligomeric organization of the alpha and beta subunits of mouse meprin A. J Biol Chem. 1994 May 27;269(21):15388–15393. [PubMed] [Google Scholar]
- Matsumura Y., Hisaki K., Takaoka M., Morimoto S. Phosphoramidon, a metalloproteinase inhibitor, suppresses the hypertensive effect of big endothelin-1. Eur J Pharmacol. 1990 Aug 21;185(1):103–106. doi: 10.1016/0014-2999(90)90216-s. [DOI] [PubMed] [Google Scholar]
- Matsumura Y., Ikegawa R., Tsukahara Y., Takaoka M., Morimoto S. Conversion of big endothelin-1 to endothelin-1 by two types of metalloproteinases derived from porcine aortic endothelial cells. FEBS Lett. 1990 Oct 15;272(1-2):166–170. doi: 10.1016/0014-5793(90)80475-x. [DOI] [PubMed] [Google Scholar]
- Matsumura Y., Ikegawa R., Tsukahara Y., Takaoka M., Morimoto S. Conversion of big endothelin-1 to endothelin-1 by two-types of metalloproteinases of cultured porcine vascular smooth muscle cells. Biochem Biophys Res Commun. 1991 Aug 15;178(3):899–905. doi: 10.1016/0006-291x(91)90976-e. [DOI] [PubMed] [Google Scholar]
- Matsumura Y., Tsukahara Y., Kuninobu K., Takaoka M., Morimoto S. Phosphoramidon-sensitive endothelin-converting enzyme in vascular endothelial cells converts big endothelin-1 and big endothelin-3 to their mature form. FEBS Lett. 1992 Jun 29;305(2):86–90. doi: 10.1016/0014-5793(92)80870-m. [DOI] [PubMed] [Google Scholar]
- McMahon E. G., Palomo M. A., Moore W. M., McDonald J. F., Stern M. K. Phosphoramidon blocks the pressor activity of porcine big endothelin-1-(1-39) in vivo and conversion of big endothelin-1-(1-39) to endothelin-1-(1-21) in vitro. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):703–707. doi: 10.1073/pnas.88.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyauchi T., Yanagisawa M., Tomizawa T., Sugishita Y., Suzuki N., Fujino M., Ajisaka R., Goto K., Masaki T. Increased plasma concentrations of endothelin-1 and big endothelin-1 in acute myocardial infarction. Lancet. 1989 Jul 1;2(8653):53–54. doi: 10.1016/s0140-6736(89)90303-6. [DOI] [PubMed] [Google Scholar]
- Ohnaka K., Takayanagi R., Nishikawa M., Haji M., Nawata H. Purification and characterization of a phosphoramidon-sensitive endothelin-converting enzyme in porcine aortic endothelium. OFF. J Biol Chem. 1993 Dec 15;268(35):26759–26766. [PubMed] [Google Scholar]
- Ohnaka K., Takayanagi R., Yamauchi T., Umeda F., Nawata H. Cultured bovine endothelial cells convert big endothelin isopeptides to mature endothelin isopeptides. Biochem Int. 1991 Feb;23(3):499–506. [PubMed] [Google Scholar]
- Oliver F. J., de la Rubia G., Feener E. P., Lee M. E., Loeken M. R., Shiba T., Quertermous T., King G. L. Stimulation of endothelin-1 gene expression by insulin in endothelial cells. J Biol Chem. 1991 Dec 5;266(34):23251–23256. [PubMed] [Google Scholar]
- Opgenorth T. J., Wu-Wong J. R., Shiosaki K. Endothelin-converting enzymes. FASEB J. 1992 Jun;6(9):2653–2659. doi: 10.1096/fasebj.6.9.1612289. [DOI] [PubMed] [Google Scholar]
- Sawamura T., Kimura S., Shinmi O., Sugita Y., Yanagisawa M., Goto K., Masaki T. Purification and characterization of putative endothelin converting enzyme in bovine adrenal medulla: evidence for a cathepsin D-like enzyme. Biochem Biophys Res Commun. 1990 May 16;168(3):1230–1236. doi: 10.1016/0006-291x(90)91160-t. [DOI] [PubMed] [Google Scholar]
- Sawamura T., Shinmi O., Kishi N., Sugita Y., Yanagisawa M., Goto K., Masaki T., Kimura S. Characterization of phosphoramidon-sensitive metalloproteinases with endothelin-converting enzyme activity in porcine lung membrane. Biochim Biophys Acta. 1993 Feb 13;1161(2-3):295–302. doi: 10.1016/0167-4838(93)90228-j. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H., Warner T. D., Ishii K., Sheng H., Murad F. Insulin secretion from pancreatic B cells caused by L-arginine-derived nitrogen oxides. Science. 1992 Feb 7;255(5045):721–723. doi: 10.1126/science.1371193. [DOI] [PubMed] [Google Scholar]
- Schmidt M., Kröger B., Jacob E., Seulberger H., Subkowski T., Otter R., Meyer T., Schmalzing G., Hillen H. Molecular characterization of human and bovine endothelin converting enzyme (ECE-1). FEBS Lett. 1994 Dec 19;356(2-3):238–243. doi: 10.1016/0014-5793(94)01277-6. [DOI] [PubMed] [Google Scholar]
- Shimada K., Matsushita Y., Wakabayashi K., Takahashi M., Matsubara A., Iijima Y., Tanzawa K. Cloning and functional expression of human endothelin-converting enzyme cDNA. Biochem Biophys Res Commun. 1995 Feb 15;207(2):807–812. doi: 10.1006/bbrc.1995.1258. [DOI] [PubMed] [Google Scholar]
- Shimada K., Takahashi M., Tanzawa K. Cloning and functional expression of endothelin-converting enzyme from rat endothelial cells. J Biol Chem. 1994 Jul 15;269(28):18275–18278. [PubMed] [Google Scholar]
- Suzuki N., Matsumoto H., Kitada C., Masaki T., Fujino M. A sensitive sandwich-enzyme immunoassay for human endothelin. J Immunol Methods. 1989 Mar 31;118(2):245–250. doi: 10.1016/0022-1759(89)90012-4. [DOI] [PubMed] [Google Scholar]
- Takada J., Okada K., Ikenaga T., Matsuyama K., Yano M. Phosphoramidon-sensitive endothelin-converting enzyme in the cytosol of cultured bovine endothelial cells. Biochem Biophys Res Commun. 1991 Apr 30;176(2):860–865. doi: 10.1016/s0006-291x(05)80265-7. [DOI] [PubMed] [Google Scholar]
- Takebe Y., Seiki M., Fujisawa J., Hoy P., Yokota K., Arai K., Yoshida M., Arai N. SR alpha promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol Cell Biol. 1988 Jan;8(1):466–472. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner A. J. PIG-tailed membrane proteins. Essays Biochem. 1994;28:113–127. [PubMed] [Google Scholar]
- Waxman L., Doshi K. P., Gaul S. L., Wang S., Bednar R. A., Stern A. M. Identification and characterization of endothelin converting activity from EAHY 926 cells: evidence for the physiologically relevant human enzyme. Arch Biochem Biophys. 1994 Jan;308(1):240–253. doi: 10.1006/abbi.1994.1034. [DOI] [PubMed] [Google Scholar]
- Williams T. A., Barnes K., Kenny A. J., Turner A. J., Hooper N. M. A comparison of the zinc contents and substrate specificities of the endothelial and testicular forms of porcine angiotensin converting enzyme and the preparation of isoenzyme-specific antisera. Biochem J. 1992 Dec 15;288(Pt 3):875–881. doi: 10.1042/bj2880875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D., Emoto N., Giaid A., Slaughter C., Kaw S., deWit D., Yanagisawa M. ECE-1: a membrane-bound metalloprotease that catalyzes the proteolytic activation of big endothelin-1. Cell. 1994 Aug 12;78(3):473–485. doi: 10.1016/0092-8674(94)90425-1. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988 Mar 31;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Masaki T. Molecular biology and biochemistry of the endothelins. Trends Pharmacol Sci. 1989 Sep;10(9):374–378. doi: 10.1016/0165-6147(89)90011-4. [DOI] [PubMed] [Google Scholar]