Abstract
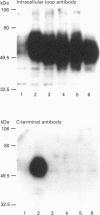
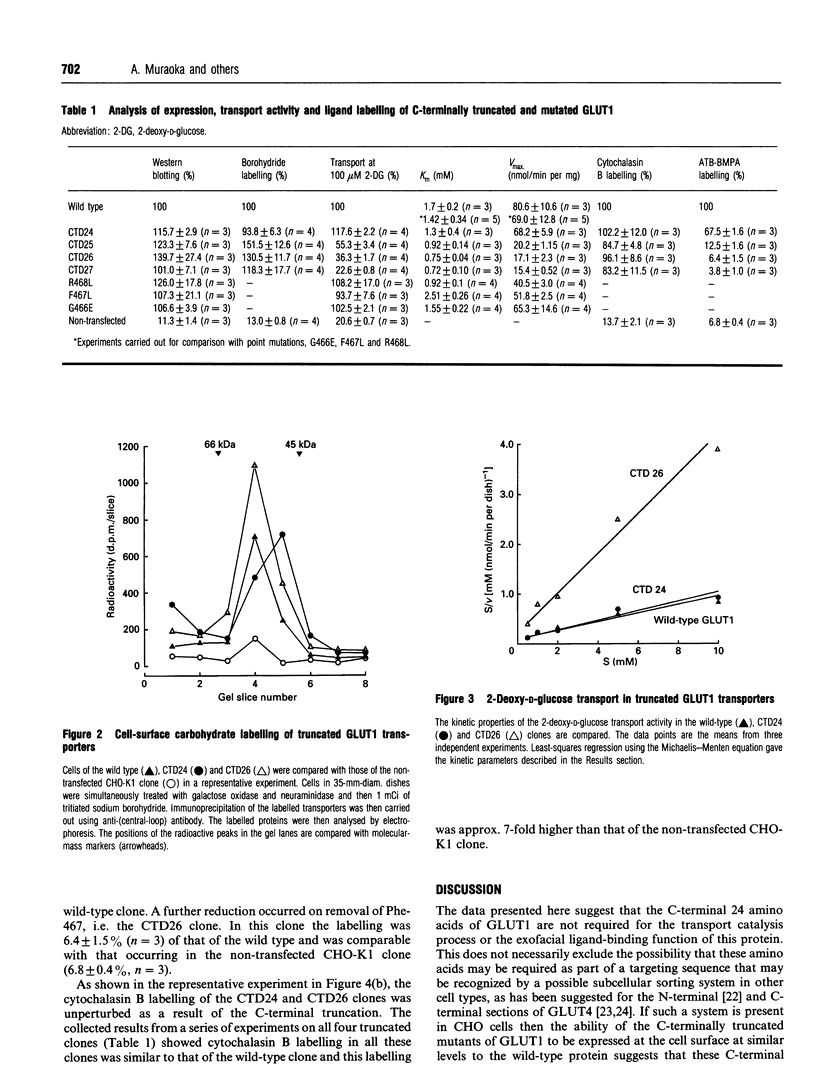
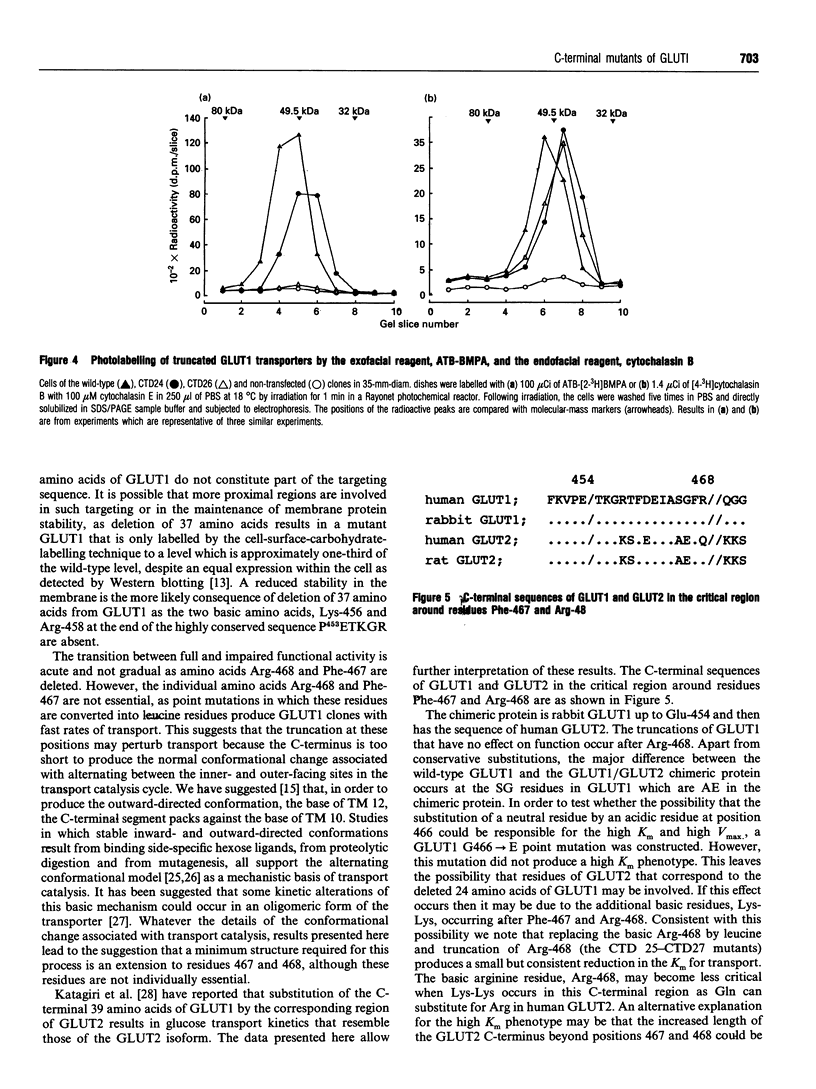
C-terminally truncated and mutated forms of GLUT1 have been constructed to determine the minimum structure at the C-terminus required for glucose transport activity and ligand binding at the outer and inner binding sites. Four truncated mutants have been constructed (CTD24 to CTD27) in which 24 to 27 amino acids are deleted. In addition, point substitutions of R468-->L, F467-->L and G466-->E have been produced. Chinese hamster ovary clones which were transfected with these mutant GLUT1s were shown, by Western blotting and cell-surface carbohydrate labelling, to have expression levels which were comparable with the wild-type clone. Wild-type levels of 2-deoxy-D-glucose transport activity were retained only in the clone transfected with the construct in which 24 amino acids were deleted (CTD24). The CTD25, CTD26 and CTD27 clones showed markedly reduced transport activity. From a kinetic comparison of the CTD24 and CTD26 clones it was found that the reduced transport was mainly associated with a reduced Vmax. value for 2-deoxy-D-glucose uptake but with a slight lowering of the Km. These data establish that the 24 amino acids at the C-terminus of GLUT1 are not required for the transport catalysis. However, the point mutations of F467L and G466E (26 and 27 residues from the C-terminus) did not significantly perturb the kinetics of 2-deoxy-D-glucose transport. The substitution of R468L produced a slight, but significant, lowering of the Km. The ability of the truncated GLUt1s to bind the exofacial ligand, 2-N-4-(1-zai-2,2,2-trifluoroethyl)benzoyl-1,3-bis-(D-mannos- 4-yl-oxy) -2-propylamine (ATB-BMPA), and the endofacial ligand, cytochalasin B, were assessed by photolabelling procedures. The ability to bind ATB-BMPA was retained only in the CTD24 truncated mutant and was reduced to levels comparable with those of the non-transfected clone in the other mutant clones. Cytochalasin B labelling was unimpaired in all four mutated GLUT1s. These data establish that a minimum structure at the C-terminus of GLUT1, which is required for the conformational change to expose the exofacial site, includes amino acids at positions Phe-467 and Arg-468; however, these amino acids are not individually essential.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleman J. R., Lienhard G. E. Rapid kinetics of the glucose transporter from human erythrocytes. Detection and measurement of a half-turnover of the purified transporter. J Biol Chem. 1985 Apr 25;260(8):4575–4578. [PubMed] [Google Scholar]
- Barnett J. E., Holman G. D., Munday K. A. Structural requirements for binding to the sugar-transport system of the human erythrocyte. Biochem J. 1973 Feb;131(2):211–221. doi: 10.1042/bj1310211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns M. T., Alvarez J., Panico M., Gibbs A. F., Morris H. R., Chapman D., Baldwin S. A. Investigation of the structure and function of the human erythrocyte glucose transporter by proteolytic dissection. Biochim Biophys Acta. 1987 Dec 11;905(2):295–310. doi: 10.1016/0005-2736(87)90458-5. [DOI] [PubMed] [Google Scholar]
- Clark A. E., Holman G. D. Exofacial photolabelling of the human erythrocyte glucose transporter with an azitrifluoroethylbenzoyl-substituted bismannose. Biochem J. 1990 Aug 1;269(3):615–622. doi: 10.1042/bj2690615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope D. L., Holman G. D., Baldwin S. A., Wolstenholme A. J. Domain assembly of the GLUT1 glucose transporter. Biochem J. 1994 Jun 1;300(Pt 2):291–294. doi: 10.1042/bj3000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czech M. P., Chawla A., Woon C. W., Buxton J., Armoni M., Tang W., Joly M., Corvera S. Exofacial epitope-tagged glucose transporter chimeras reveal COOH-terminal sequences governing cellular localization. J Cell Biol. 1993 Oct;123(1):127–135. doi: 10.1083/jcb.123.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deziel M., Pegg W., Mack E., Rothstein A., Klip A. Labelling of the human erythrocyte glucose transporter with 3H-labelled cytochalasin B occurs via protein photoactivation. Biochim Biophys Acta. 1984 May 30;772(3):403–406. doi: 10.1016/0005-2736(84)90157-3. [DOI] [PubMed] [Google Scholar]
- Gould G. W., Holman G. D. The glucose transporter family: structure, function and tissue-specific expression. Biochem J. 1993 Oct 15;295(Pt 2):329–341. doi: 10.1042/bj2950329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashiramoto M., Kadowaki T., Clark A. E., Muraoka A., Momomura K., Sakura H., Tobe K., Akanuma Y., Yazaki Y., Holman G. D. Site-directed mutagenesis of GLUT1 in helix 7 residue 282 results in perturbation of exofacial ligand binding. J Biol Chem. 1992 Sep 5;267(25):17502–17507. [PubMed] [Google Scholar]
- Hebert D. N., Carruthers A. Glucose transporter oligomeric structure determines transporter function. Reversible redox-dependent interconversions of tetrameric and dimeric GLUT1. J Biol Chem. 1992 Nov 25;267(33):23829–23838. [PubMed] [Google Scholar]
- Henderson P. J. The homologous glucose transport proteins of prokaryotes and eukaryotes. Res Microbiol. 1990 Mar-Apr;141(3):316–328. doi: 10.1016/0923-2508(90)90005-b. [DOI] [PubMed] [Google Scholar]
- Holman G. D., Rees W. D. Photolabelling of the hexose transporter at external and internal sites: fragmentation patterns and evidence for a conformational change. Biochim Biophys Acta. 1987 Mar 12;897(3):395–405. doi: 10.1016/0005-2736(87)90437-8. [DOI] [PubMed] [Google Scholar]
- Hresko R. C., Kruse M., Strube M., Mueckler M. Topology of the Glut 1 glucose transporter deduced from glycosylation scanning mutagenesis. J Biol Chem. 1994 Aug 12;269(32):20482–20488. [PubMed] [Google Scholar]
- Kaback H. R. In and out and up and down with lac permease. Int Rev Cytol. 1992;137:97–125. doi: 10.1016/s0074-7696(08)62674-1. [DOI] [PubMed] [Google Scholar]
- Katagiri H., Asano T., Ishihara H., Tsukuda K., Lin J. L., Inukai K., Kikuchi M., Yazaki Y., Oka Y. Replacement of intracellular C-terminal domain of GLUT1 glucose transporter with that of GLUT2 increases Vmax and Km of transport activity. J Biol Chem. 1992 Nov 5;267(31):22550–22555. [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin J. L., Asano T., Katagiri H., Tsukuda K., Ishihara H., Inukai K., Yazaki Y., Oka Y. Deletion of C-terminal 12 amino acids of GLUT1 protein does not abolish the transport activity. Biochem Biophys Res Commun. 1992 Apr 30;184(2):865–870. doi: 10.1016/0006-291x(92)90670-g. [DOI] [PubMed] [Google Scholar]
- Mori H., Hashiramoto M., Clark A. E., Yang J., Muraoka A., Tamori Y., Kasuga M., Holman G. D. Substitution of tyrosine 293 of GLUT1 locks the transporter into an outward facing conformation. J Biol Chem. 1994 Apr 15;269(15):11578–11583. [PubMed] [Google Scholar]
- Mueckler M., Caruso C., Baldwin S. A., Panico M., Blench I., Morris H. R., Allard W. J., Lienhard G. E., Lodish H. F. Sequence and structure of a human glucose transporter. Science. 1985 Sep 6;229(4717):941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- Mueckler M., Weng W., Kruse M. Glutamine 161 of Glut1 glucose transporter is critical for transport activity and exofacial ligand binding. J Biol Chem. 1994 Aug 12;269(32):20533–20538. [PubMed] [Google Scholar]
- Piper R. C., Tai C., Slot J. W., Hahn C. S., Rice C. M., Huang H., James D. E. The efficient intracellular sequestration of the insulin-regulatable glucose transporter (GLUT-4) is conferred by the NH2 terminus. J Cell Biol. 1992 May;117(4):729–743. doi: 10.1083/jcb.117.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schürmann A., Keller K., Monden I., Brown F. M., Wandel S., Shanahan M. F., Joost H. G. Glucose transport activity and photolabelling with 3-[125I]iodo-4-azidophenethylamido-7-O-succinyldeacetyl (IAPS)-forskolin of two mutants at tryptophan-388 and -412 of the glucose transporter GLUT1: dissociation of the binding domains of forskolin and glucose. Biochem J. 1993 Mar 1;290(Pt 2):497–501. doi: 10.1042/bj2900497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamori Y., Hashiramoto M., Clark A. E., Mori H., Muraoka A., Kadowaki T., Holman G. D., Kasuga M. Substitution at Pro385 of GLUT1 perturbs the glucose transport function by reducing conformational flexibility. J Biol Chem. 1994 Jan 28;269(4):2982–2986. [PubMed] [Google Scholar]
- Verhey K. J., Hausdorff S. F., Birnbaum M. J. Identification of the carboxy terminus as important for the isoform-specific subcellular targeting of glucose transporter proteins. J Cell Biol. 1993 Oct;123(1):137–147. doi: 10.1083/jcb.123.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadzinski B. E., Shanahan M. F., Clark R. B., Ruoho A. E. Identification of the glucose transporter in mammalian cell membranes with a 125I-forskolin photoaffinity label. Biochem J. 1988 Nov 1;255(3):983–990. doi: 10.1042/bj2550983. [DOI] [PMC free article] [PubMed] [Google Scholar]