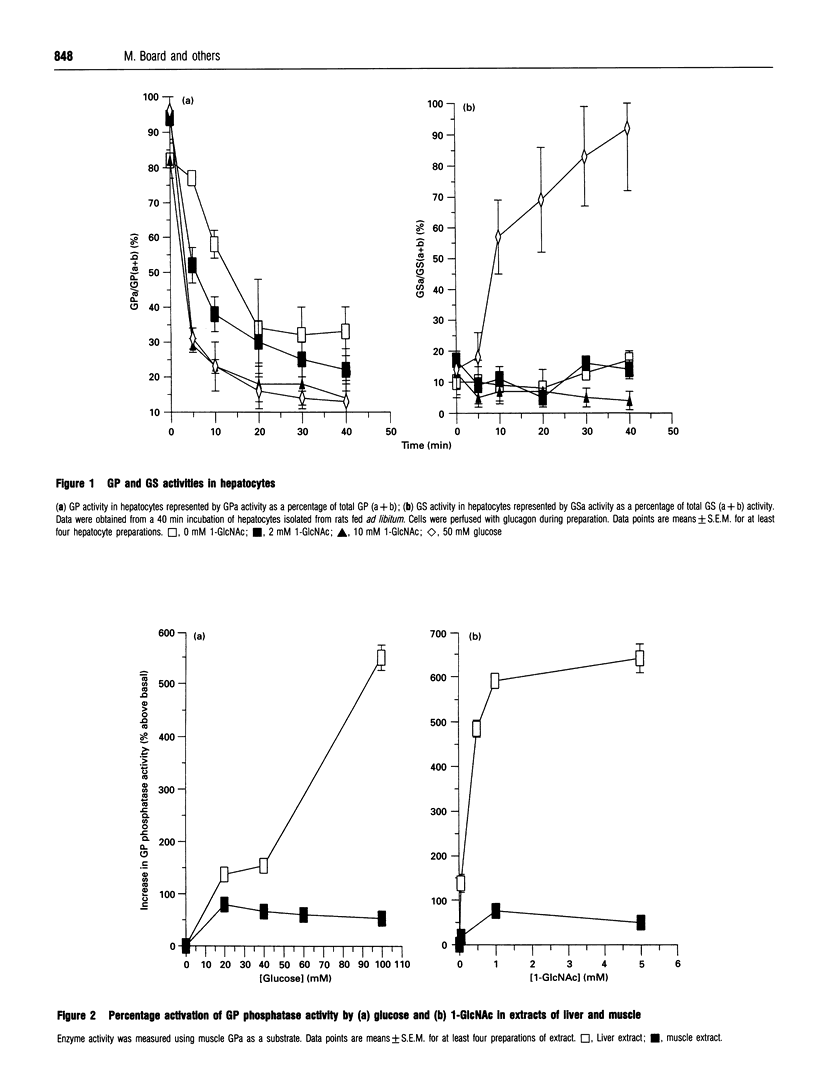
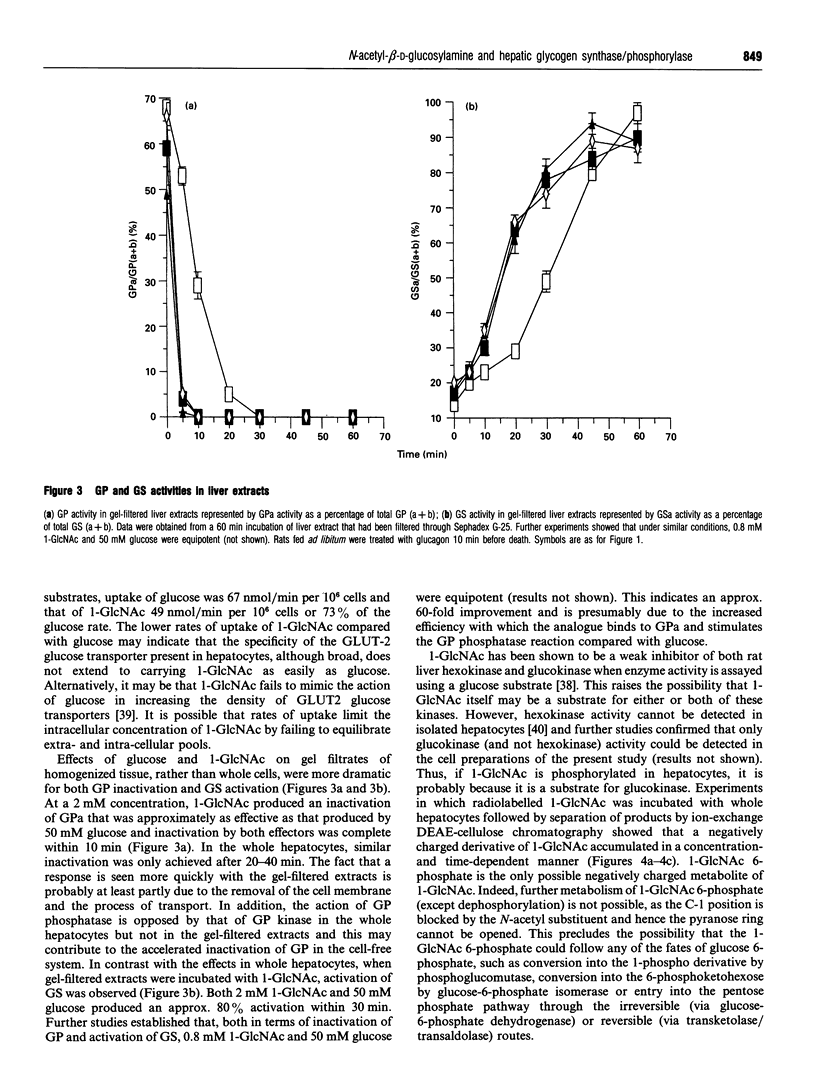
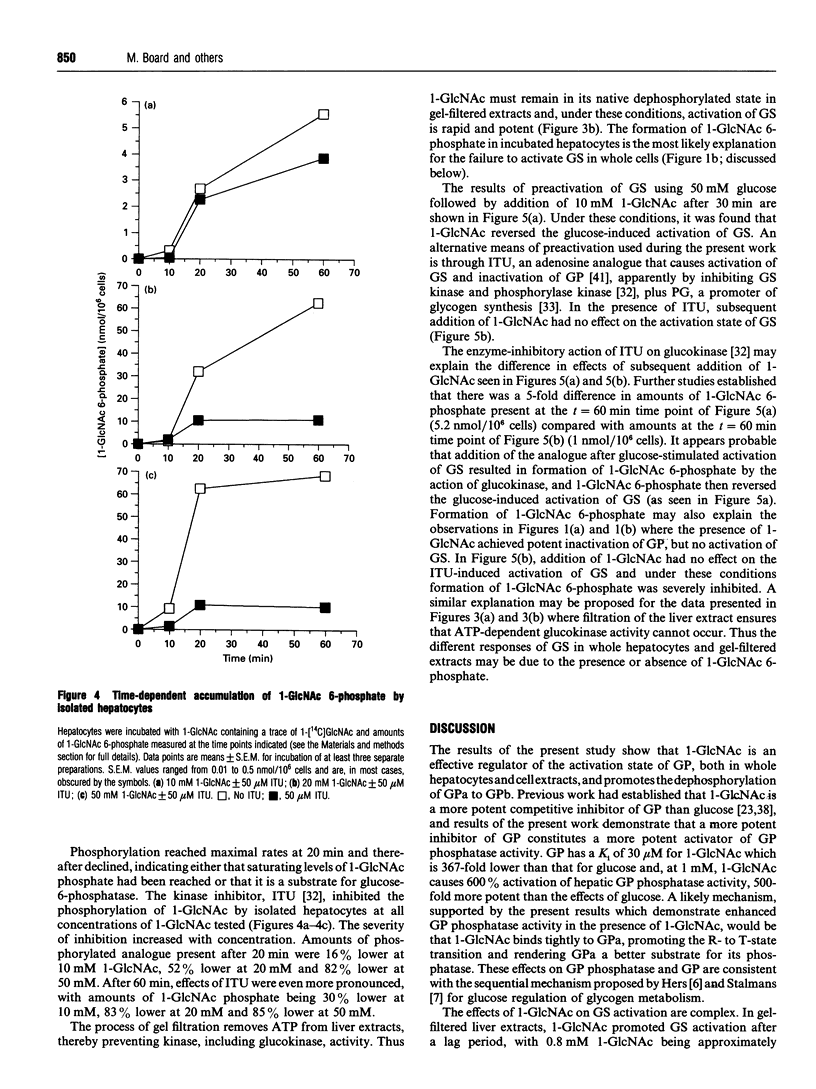
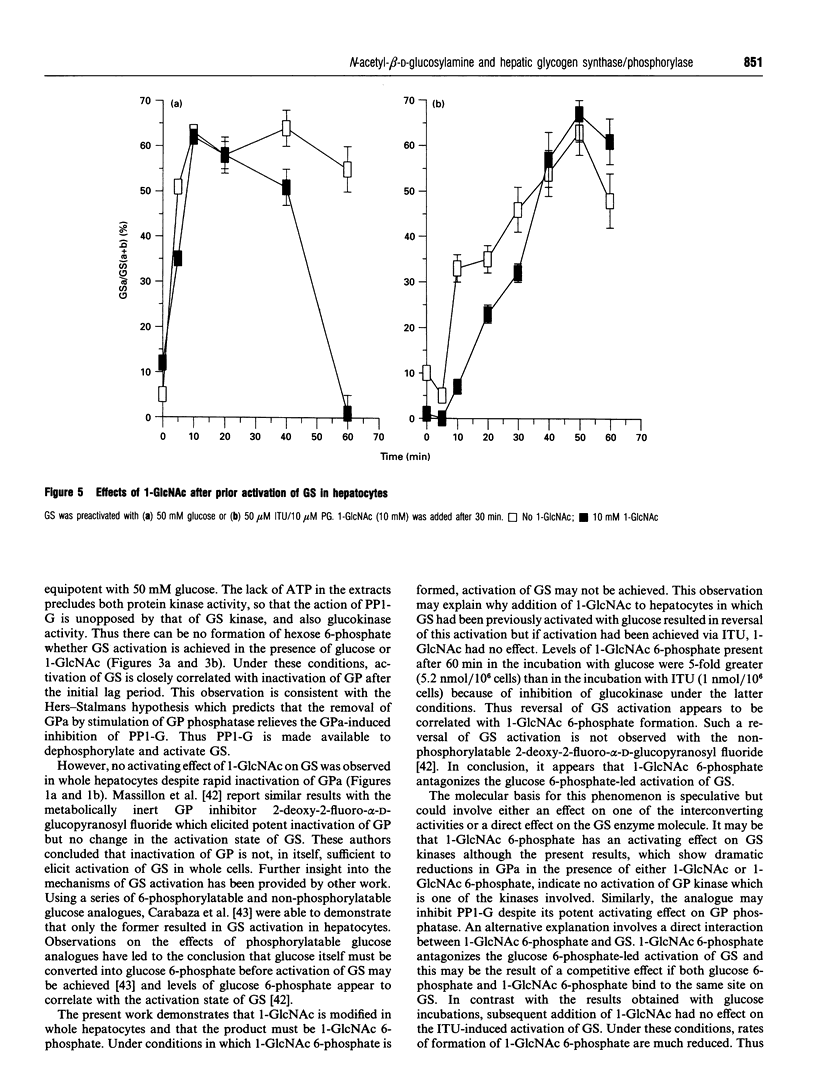
Abstract
A series of glucose-analogue inhibitors of glycogen phosphorylase b (GPb) has been designed, synthesized and investigated in crystallographic binding and kinetic studies. The aim is to produce a compound that may exert more effective control over glycogen metabolism than the parent glucose molecule and which could alleviate hyperglycaemia in Type-II diabetes. N-Acetyl-beta-D-glucopyranosylamine (1-GlcNAc) has a Ki for muscle GPb in crude extracts of 30 microM, 367-fold lower than that of beta-D-glucose [Board, Hadwen and Johnson (1995) Eur. J. Biochem. 228, 753-761]. In the current work, the effects of 1-GlcNAc on the activation states of GP and glycogen synthase (GS) in cell-free preparations and in isolated hepatocytes are reported. In gel-filtered extracts of liver, which lack ATP for kinase activity, 1-GlcNAc produced a rapid and time-dependent inactivation of GP with a subsequent activation of GS. Effects of 1-GlcNAc on both enzymes were stronger than those of glucose, with 0.8 mM 1-GlcNAc being equipotent with 50 mM glucose. At 1 mM, 1-GlcNAc enhanced the dephosphorylation of exogenous GPa by liver extracts (600%) and by muscle extracts (75%). This represents an approximately 500-fold improvement on glucose for the liver activity and 40-fold for the muscle activity. In whole hepatocytes, 1-GlcNAc showed an approximately 5-fold enhancement of glucose effects for GP inactivation but failed to elicit activation of GS. Glucose-induced activation of GS in whole hepatocytes was reversed by subsequent addition of 1-GlcNAc. However, when GS activation was achieved via the adenosine analogue and kinase inhibitor, 5'-iodotubercidin (ITU), subsequent addition of 1-GlcNAc allowed continued activation of GS. Phosphorylation of 1-GlcNAc in rat hepatocytes was established using radiolabelled material. The rate of phosphorylation was 1.60 nmol/min per 10(6) cells at 20 mM 1-GlcNAc but was reduced by the presence of 50 microM ITU (0.775 nmol/min per 10(6) cells). It is suggested that the phosphorylated derivative of 1-GlcNAc formed in hepatocytes is 1-GlcNAc 6-phosphate and that the presence of this species is responsible for the failure of 1-GlcNAc to activate GS. The relative importance of the reduction in concentration of GPa versus increased glucose 6-phosphate levels for activation of GS is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alemany S., Cohen P. Phosphorylase a is an allosteric inhibitor of the glycogen and microsomal forms of rat hepatic protein phosphatase-1. FEBS Lett. 1986 Mar 31;198(2):194–202. doi: 10.1016/0014-5793(86)80404-5. [DOI] [PubMed] [Google Scholar]
- Antoniw J. F., Nimmo H. G., Yeaman S. J., Cohen P. Comparison of the substrate specificities of protein phosphatases involved in the regulation of glycogen metabolism in rabbit skeletal muscle. Biochem J. 1977 Feb 15;162(2):423–433. doi: 10.1042/bj1620423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai G., Zhang Z. J., Werner R., Nuttall F. Q., Tan A. W., Lee E. Y. The primary structure of rat liver glycogen synthase deduced by cDNA cloning. Absence of phosphorylation sites 1a and 1b. J Biol Chem. 1990 May 15;265(14):7843–7848. [PubMed] [Google Scholar]
- Board M., Hadwen M., Johnson L. N. Effects of novel analogues of D-glucose on glycogen phosphorylase activities in crude extracts of liver and skeletal muscle. Eur J Biochem. 1995 Mar 15;228(3):753–761. doi: 10.1111/j.1432-1033.1995.0753m.x. [DOI] [PubMed] [Google Scholar]
- Bollen M., Hue L., Stalmans W. Effects of glucose on phosphorylase and glycogen synthase in hepatocytes from diabetic rats. Biochem J. 1983 Mar 15;210(3):783–787. doi: 10.1042/bj2100783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollen M., Stalmans W. The structure, role, and regulation of type 1 protein phosphatases. Crit Rev Biochem Mol Biol. 1992;27(3):227–281. doi: 10.3109/10409239209082564. [DOI] [PubMed] [Google Scholar]
- Carabaza A., Ciudad C. J., Baqué S., Guinovart J. J. Glucose has to be phosphorylated to activate glycogen synthase, but not to inactivate glycogen phosphorylase in hepatocytes. FEBS Lett. 1992 Jan 20;296(2):211–214. doi: 10.1016/0014-5793(92)80381-p. [DOI] [PubMed] [Google Scholar]
- Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- Crisp D. M., Pogson C. I. Glycolytic and gluconeogenic enzyme activities in parenchymal and non-parenchymal cells from mouse liver. Biochem J. 1972 Feb;126(4):1009–1023. doi: 10.1042/bj1261009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent P., Lavoinne A., Nakielny S., Caudwell F. B., Watt P., Cohen P. The molecular mechanism by which insulin stimulates glycogen synthesis in mammalian skeletal muscle. Nature. 1990 Nov 22;348(6299):302–308. doi: 10.1038/348302a0. [DOI] [PubMed] [Google Scholar]
- Detwiler T. C., Gratecos D., Fischer E. H. Rabbit muscle phosphorylase phosphatase. 2. Kinetic properties and behavior in glycogen particles. Biochemistry. 1977 Nov 1;16(22):4818–4823. doi: 10.1021/bi00641a010. [DOI] [PubMed] [Google Scholar]
- Fletterick R. J., Madsen N. B. The structures and related functions of phosphorylase a. Annu Rev Biochem. 1980;49:31–61. doi: 10.1146/annurev.bi.49.070180.000335. [DOI] [PubMed] [Google Scholar]
- Flückiger-Isler R. E., Walter P. Stimulation of rat liver glycogen synthesis by the adenosine kinase inhibitor 5-iodotubercidin. Biochem J. 1993 May 15;292(Pt 1):85–91. doi: 10.1042/bj2920085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R. A., Yamanouchi K., Roach P. J., Yen T. T., Dominianni S. J., Stephens T. W. Stabilization of glycogen stores and stimulation of glycogen synthesis in hepatocytes by phenacyl imidazolium compounds. J Biol Chem. 1989 Sep 5;264(25):14674–14680. [PubMed] [Google Scholar]
- Hers H. G. The control of glycogen metabolism in the liver. Annu Rev Biochem. 1976;45:167–189. doi: 10.1146/annurev.bi.45.070176.001123. [DOI] [PubMed] [Google Scholar]
- Hue L., Bontemps F., Hers H. The effects of glucose and of potassium ions on the interconversion of the two forms of glycogen phosphorylase and of glycogen synthetase in isolated rat liver preparations. Biochem J. 1975 Oct;152(1):105–114. doi: 10.1042/bj1520105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Martensen T. M., Brotherton J. E., Graves D. J. Kinetic studies of the activation of muscle phosphorylase phosphatase. J Biol Chem. 1973 Dec 25;248(24):8329–8336. [PubMed] [Google Scholar]
- Martin J. L., Veluraja K., Ross K., Johnson L. N., Fleet G. W., Ramsden N. G., Bruce I., Orchard M. G., Oikonomakos N. G., Papageorgiou A. C. Glucose analogue inhibitors of glycogen phosphorylase: the design of potential drugs for diabetes. Biochemistry. 1991 Oct 22;30(42):10101–10116. doi: 10.1021/bi00106a006. [DOI] [PubMed] [Google Scholar]
- Massillon D., Stalmans W., van de Werve G., Bollen M. Identification of the glycogenic compound 5-iodotubercidin as a general protein kinase inhibitor. Biochem J. 1994 Apr 1;299(Pt 1):123–128. doi: 10.1042/bj2990123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto K., Hase K., Takagi T., Fujii T., Taketani Y., Minami H., Oka T., Nakabou Y. Differential responses of intestinal glucose transporter mRNA transcripts to levels of dietary sugars. Biochem J. 1993 Oct 1;295(Pt 1):211–215. doi: 10.1042/bj2950211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mvumbi L., Doperé F., Stalmans W. The inhibitory effect of phosphorylase a on the activation of glycogen synthase depends on the type of synthase phosphatase. Biochem J. 1983 May 15;212(2):407–416. doi: 10.1042/bj2120407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakielny S., Campbell D. G., Cohen P. The molecular mechanism by which adrenalin inhibits glycogen synthesis. Eur J Biochem. 1991 Aug 1;199(3):713–722. doi: 10.1111/j.1432-1033.1991.tb16175.x. [DOI] [PubMed] [Google Scholar]
- Nimmo G. A., Cohen P. The regulation of glycogen metabolism. Purification and characterisation of protein phosphatase inhibitor-1 from rabbit skeletal muscle. Eur J Biochem. 1978 Jun 15;87(2):341–351. doi: 10.1111/j.1432-1033.1978.tb12383.x. [DOI] [PubMed] [Google Scholar]
- Nyomba B. L., Brautigan D. L., Schlender K. K., Wang W., Bogardus C., Mott D. M. Deficiency in phosphorylase phosphatase activity despite elevated protein phosphatase type-1 catalytic subunit in skeletal muscle from insulin-resistant subjects. J Clin Invest. 1991 Nov;88(5):1540–1545. doi: 10.1172/JCI115464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulter L., Ang S. G., Gibson B. W., Williams D. H., Holmes C. F., Caudwell F. B., Pitcher J., Cohen P. Analysis of the in vivo phosphorylation state of rabbit skeletal muscle glycogen synthase by fast-atom-bombardment mass spectrometry. Eur J Biochem. 1988 Aug 15;175(3):497–510. doi: 10.1111/j.1432-1033.1988.tb14222.x. [DOI] [PubMed] [Google Scholar]
- Sprang S. R., Goldsmith E. J., Fletterick R. J., Withers S. G., Madsen N. B. Catalytic site of glycogen phosphorylase: structure of the T state and specificity for alpha-D-glucose. Biochemistry. 1982 Oct 12;21(21):5364–5371. doi: 10.1021/bi00264a038. [DOI] [PubMed] [Google Scholar]
- Stalmans W., De Wulf H., Hue L., Hers H. G. The sequential inactivation of glycogen phosphorylase and activation of glycogen synthetase in liver after the administration of glucose to mice and rats. The mechanism of the hepatic threshold to glucose. Eur J Biochem. 1974 Jan 3;41(1):127–134. doi: 10.1111/j.1432-1033.1974.tb03252.x. [DOI] [PubMed] [Google Scholar]
- Stalmans W. The role of the liver in the homeostasis of blood glucose. Curr Top Cell Regul. 1976;11:51–97. doi: 10.1016/b978-0-12-152811-9.50009-2. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Schlender K. K., Larner J. A rapid filter paper assay for UDPglucose-glycogen glucosyltransferase, including an improved biosynthesis of UDP-14C-glucose. Anal Biochem. 1968 Oct 24;25(1):486–499. doi: 10.1016/0003-2697(68)90127-9. [DOI] [PubMed] [Google Scholar]
- Uyttenhove K., Bollen M., Stalmans W. An optimized assay of phosphorylase kinase in crude liver preparations. Biochem J. 1991 Sep 15;278(Pt 3):899–901. doi: 10.1042/bj2780899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandebroeck A., Bollen M., De Wulf H., Stalmans W. An assessment of the importance of intralysosomal and of alpha-amylolytic glycogenolysis in the liver of normal rats and of rats with a glycogen-storage disease. Eur J Biochem. 1985 Dec 16;153(3):621–628. doi: 10.1111/j.1432-1033.1985.tb09345.x. [DOI] [PubMed] [Google Scholar]
- Vandenheede J. R., Keppens S., De Wulf H. The activation of liver phosphorylase b kinase by glucagon. FEBS Lett. 1976 Jan 15;61(2):213–217. doi: 10.1016/0014-5793(76)81040-x. [DOI] [PubMed] [Google Scholar]
- Watson K. A., Mitchell E. P., Johnson L. N., Son J. C., Bichard C. J., Orchard M. G., Fleet G. W., Oikonomakos N. G., Leonidas D. D., Kontou M. Design of inhibitors of glycogen phosphorylase: a study of alpha- and beta-C-glucosides and 1-thio-beta-D-glucose compounds. Biochemistry. 1994 May 17;33(19):5745–5758. doi: 10.1021/bi00185a011. [DOI] [PubMed] [Google Scholar]
- Wera S., Bollen M., Stalmans W. Purification and characterization of the glycogen-bound protein phosphatase from rat liver. J Biol Chem. 1991 Jan 5;266(1):339–345. [PubMed] [Google Scholar]
- Witters L. A., Avruch J. Insulin regulation of hepatic glycogen synthase and phosphorylase. Biochemistry. 1978 Feb 7;17(3):406–410. doi: 10.1021/bi00596a004. [DOI] [PubMed] [Google Scholar]


