Abstract
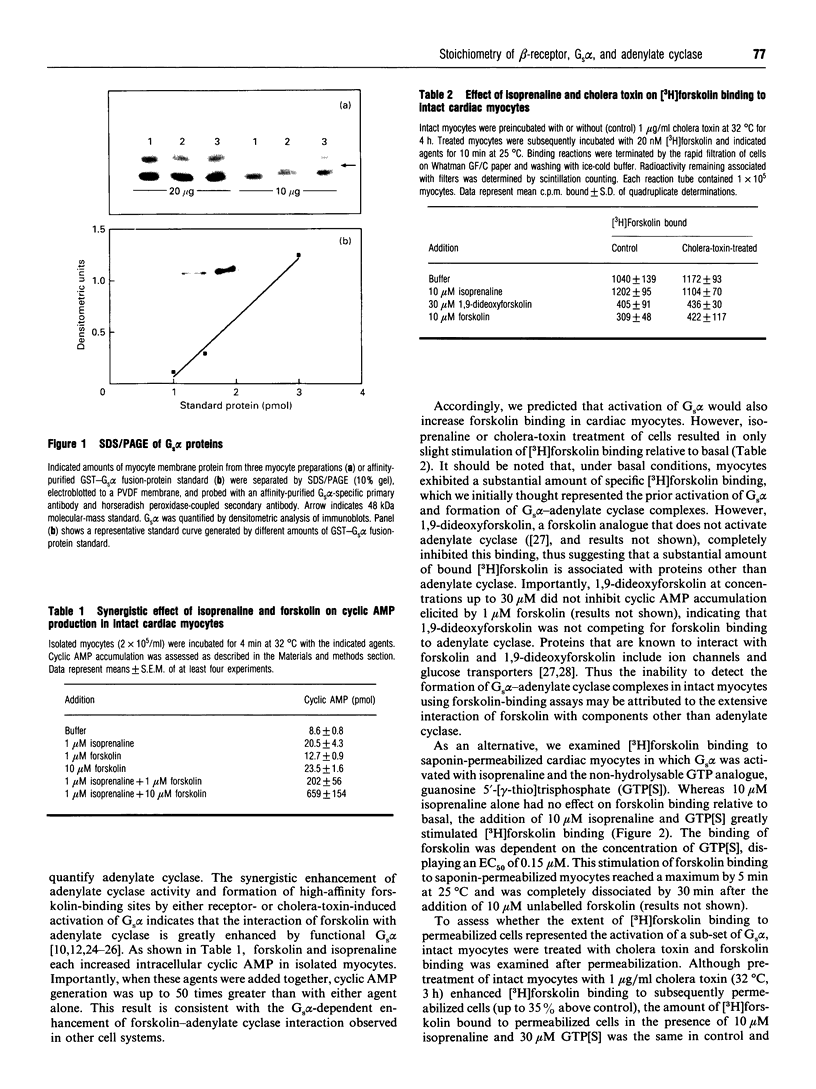
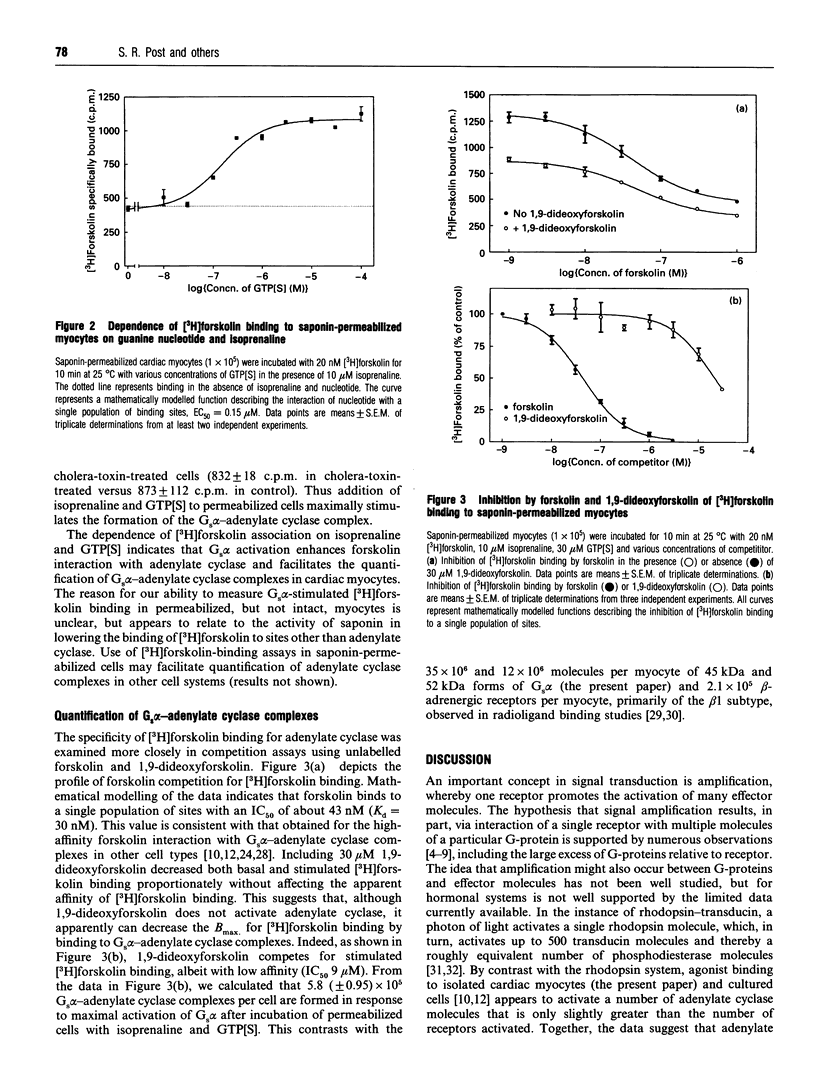
We have investigated the stoichiometric relationship of proteins involved in beta-adrenergic-receptor-mediated signal transduction in isolated rat cardiac myocytes. These cells contain about 2.1 x 10(5) beta-adrenergic receptors per cell, as determined by radio-ligand-binding assays. We have assessed the amount of Gs alpha present in myocyte membranes by immunoblotting using a purified glutathione S-transferase-Gs alpha fusion protein as a standard for quantification. By this method, we determined that cardiac myocytes contain about 35 x 10(6) and 12 x 10(6) molecules per cell of the 45 and 52 kDa forms of Gs alpha, respectively. [3H]Forskolin binding assays were used to assess the formation of high-affinity forskolin binding sites representing Gs alpha-adenylate cyclase complexes occurring in response to Gs alpha activation. Quantification of the adenylate cyclase complexes was facilitated by the permeabilization of cells with saponin. The addition of isoprenaline (isoproterenol) and guanosine 5'-[gamma-thio]trisphosphate to saponin-permeabilized myocytes results in the formation of 6 x 10(5) Gs alpha-adenylate cyclase complexes. Taken together, the data presented here demonstrate that, in a physiologically relevant setting, G-protein is present in large stoichiometric excess relative to both receptor and effector. In addition, we show that, overall, only modest signal amplification occurs between receptor and adenylate cyclase. Thus adenylate cyclase (rather than Gs) is the component distal to receptor that limits agonist-mediated increases in cyclic AMP production. Although limited data are as yet available for other G-protein-regulated effectors, we hypothesize that the stoichiometry of signalling components and the extent of signal amplification described for the beta-adrenergic response pathway will be applicable to other G-protein-coupled hormone receptor systems.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson S. N., Martin M. W., Hughes A. R., Harden T. K., Neve K. A., Barrett D. A., Molinoff P. B. Interaction of beta-adrenergic receptors with the inhibitory guanine nucleotide-binding protein of adenylate cyclase in membranes prepared from cyc- S49 lymphoma cells. Biochem Pharmacol. 1988 Nov 15;37(22):4289–4297. doi: 10.1016/0006-2952(88)90609-0. [DOI] [PubMed] [Google Scholar]
- Alousi A. A., Jasper J. R., Insel P. A., Motulsky H. J. Stoichiometry of receptor-Gs-adenylate cyclase interactions. FASEB J. 1991 Jun;5(9):2300–2303. doi: 10.1096/fasebj.5.9.1650314. [DOI] [PubMed] [Google Scholar]
- Barber R. Forskolin binding to intact S49 lymphoma cells. Second Messengers Phosphoproteins. 1988;12(1):59–71. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brandt D. R., Ross E. M. Catecholamine-stimulated GTPase cycle. Multiple sites of regulation by beta-adrenergic receptor and Mg2+ studied in reconstituted receptor-Gs vesicles. J Biol Chem. 1986 Feb 5;261(4):1656–1664. [PubMed] [Google Scholar]
- Buxton I. L., Brunton L. L. Compartments of cyclic AMP and protein kinase in mammalian cardiomyocytes. J Biol Chem. 1983 Sep 10;258(17):10233–10239. [PubMed] [Google Scholar]
- Buxton I. L., Brunton L. L. Direct analysis of beta-adrenergic receptor subtypes on intact adult ventricular myocytes of the rat. Circ Res. 1985 Jan;56(1):126–132. doi: 10.1161/01.res.56.1.126. [DOI] [PubMed] [Google Scholar]
- Clark R. B., Goka T. J., Green D. A., Barber R., Butcher R. W. Differences in the forskolin activation of adenylate cyclases in wild-type and variant lymphoma cells. Mol Pharmacol. 1982 Nov;22(3):609–613. [PubMed] [Google Scholar]
- Darfler F. J., Mahan L. C., Koachman A. M., Insel P. A. Stimulation of forskolin of intact S49 lymphoma cells involves the nucleotide regulatory protein of adenylate cyclase. J Biol Chem. 1982 Oct 25;257(20):11901–11907. [PubMed] [Google Scholar]
- Delemer B., Dib K., Patey M., Jacquemin C., Corrèze C. Modification of the amounts of G proteins and of the activity of adenylyl cyclase in human benign thyroid tumours. J Endocrinol. 1992 Mar;132(3):477–485. doi: 10.1677/joe.0.1320477. [DOI] [PubMed] [Google Scholar]
- Doyle D. D., Winter A. Isolation of membranes enriched in "tetrodotoxin-insensitive" saxitoxin-binding sites from mammalian ventricle. Receptor solubilization. J Biol Chem. 1989 Mar 5;264(7):3811–3817. [PubMed] [Google Scholar]
- Freissmuth M., Casey P. J., Gilman A. G. G proteins control diverse pathways of transmembrane signaling. FASEB J. 1989 Aug;3(10):2125–2131. [PubMed] [Google Scholar]
- Gao B. N., Gilman A. G. Cloning and expression of a widely distributed (type IV) adenylyl cyclase. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10178–10182. doi: 10.1073/pnas.88.22.10178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudin C., Ishikawa Y., Wight D. C., Mahdavi V., Nadal-Ginard B., Wagner T. E., Vatner D. E., Homcy C. J. Overexpression of Gs alpha protein in the hearts of transgenic mice. J Clin Invest. 1995 Apr;95(4):1676–1683. doi: 10.1172/JCI117843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gengo P. J., Bowling N., Wyss V. L., Hayes J. S. Effects of prolonged phenylephrine infusion on cardiac adrenoceptors and calcium channels. J Pharmacol Exp Ther. 1988 Jan;244(1):100–105. [PubMed] [Google Scholar]
- Gierschik P., Moghtader R., Straub C., Dieterich K., Jakobs K. H. Signal amplification in HL-60 granulocytes. Evidence that the chemotactic peptide receptor catalytically activates guanine-nucleotide-binding regulatory proteins in native plasma membranes. Eur J Biochem. 1991 May 8;197(3):725–732. doi: 10.1111/j.1432-1033.1991.tb15964.x. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Hallaq H., Smith T. W., Leaf A. Modulation of dihydropyridine-sensitive calcium channels in heart cells by fish oil fatty acids. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1760–1764. doi: 10.1073/pnas.89.5.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond H. K., Roth D. A., McKirnan M. D., Ping P. Regional myocardial downregulation of the inhibitory guanosine triphosphate-binding protein (Gi alpha 2) and beta-adrenergic receptors in a porcine model of chronic episodic myocardial ischemia. J Clin Invest. 1993 Dec;92(6):2644–2652. doi: 10.1172/JCI116880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R. J., Howard M. J., Allen J. M., Insel P. A. Decreased beta 2-adrenergic receptor mRNA expression in receptor-deficient S49 lymphoma cells. Mol Pharmacol. 1991 Dec;40(6):974–979. [PubMed] [Google Scholar]
- Kawai Y., Arinze I. J. Ontogeny of guanine-nucleotide-binding regulatory proteins in rabbit liver. Biochem J. 1991 Mar 1;274(Pt 2):439–444. doi: 10.1042/bj2740439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G. D., Adie E. J., Milligan G. Quantitative stoichiometry of the proteins of the stimulatory arm of the adenylyl cyclase cascade in neuroblastoma x glioma hybrid, NG108-15 cells. Eur J Biochem. 1994 Jan 15;219(1-2):135–143. doi: 10.1111/j.1432-1033.1994.tb19923.x. [DOI] [PubMed] [Google Scholar]
- Kuznetsov V., Pak E., Robinson R. B., Steinberg S. F. Beta 2-adrenergic receptor actions in neonatal and adult rat ventricular myocytes. Circ Res. 1995 Jan;76(1):40–52. doi: 10.1161/01.res.76.1.40. [DOI] [PubMed] [Google Scholar]
- Kwok-Keung Fung B., Stryer L. Photolyzed rhodopsin catalyzes the exchange of GTP for bound GDP in retinal rod outer segments. Proc Natl Acad Sci U S A. 1980 May;77(5):2500–2504. doi: 10.1073/pnas.77.5.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurenza A., Seamon K. B. High-affinity binding sites for [3H]forskolin. Methods Enzymol. 1991;195:52–65. doi: 10.1016/0076-6879(91)95154-c. [DOI] [PubMed] [Google Scholar]
- Laurenza A., Sutkowski E. M., Seamon K. B. Forskolin: a specific stimulator of adenylyl cyclase or a diterpene with multiple sites of action? Trends Pharmacol Sci. 1989 Nov;10(11):442–447. doi: 10.1016/S0165-6147(89)80008-2. [DOI] [PubMed] [Google Scholar]
- Matesic D. F., Manning D. R., Luthin G. R. Tissue-dependent association of muscarinic acetylcholine receptors with guanine nucleotide-binding regulatory proteins. Mol Pharmacol. 1991 Sep;40(3):347–353. [PubMed] [Google Scholar]
- Mattera R., Graziano M. P., Yatani A., Zhou Z., Graf R., Codina J., Birnbaumer L., Gilman A. G., Brown A. M. Splice variants of the alpha subunit of the G protein Gs activate both adenylyl cyclase and calcium channels. Science. 1989 Feb 10;243(4892):804–807. doi: 10.1126/science.2536957. [DOI] [PubMed] [Google Scholar]
- Milano C. A., Allen L. F., Rockman H. A., Dolber P. C., McMinn T. R., Chien K. R., Johnson T. D., Bond R. A., Lefkowitz R. J. Enhanced myocardial function in transgenic mice overexpressing the beta 2-adrenergic receptor. Science. 1994 Apr 22;264(5158):582–586. doi: 10.1126/science.8160017. [DOI] [PubMed] [Google Scholar]
- Neubig R. R. Membrane organization in G-protein mechanisms. FASEB J. 1994 Sep;8(12):939–946. doi: 10.1096/fasebj.8.12.8088459. [DOI] [PubMed] [Google Scholar]
- Offermanns S., Wieland T., Homann D., Sandmann J., Bombien E., Spicher K., Schultz G., Jakobs K. H. Transfected muscarinic acetylcholine receptors selectively couple to Gi-type G proteins and Gq/11. Mol Pharmacol. 1994 May;45(5):890–898. [PubMed] [Google Scholar]
- Pedersen S. E., Ross E. M. Functional reconstitution of beta-adrenergic receptors and the stimulatory GTP-binding protein of adenylate cyclase. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7228–7232. doi: 10.1073/pnas.79.23.7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransnäs L. A., Insel P. A. Quantitation of the guanine nucleotide binding regulatory protein Gs in S49 cell membranes using antipeptide antibodies to alpha s. J Biol Chem. 1988 Jul 5;263(19):9482–9485. [PubMed] [Google Scholar]
- Ransnäs L. A., Insel P. A. Subunit dissociation is the mechanism for hormonal activation of the Gs protein in native membranes. J Biol Chem. 1988 Nov 25;263(33):17239–17242. [PubMed] [Google Scholar]
- Roth D. A., Urasawa K., Helmer G. A., Hammond H. K. Downregulation of cardiac guanosine 5'-triphosphate-binding proteins in right atrium and left ventricle in pacing-induced congestive heart failure. J Clin Invest. 1993 Mar;91(3):939–949. doi: 10.1172/JCI116315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert B., VanDongen A. M., Kirsch G. E., Brown A. M. Beta-adrenergic inhibition of cardiac sodium channels by dual G-protein pathways. Science. 1989 Aug 4;245(4917):516–519. doi: 10.1126/science.2547248. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Vaillancourt R., Daly J. W. Modulation of forskolin binding to rat brain membranes. J Cyclic Nucleotide Protein Phosphor Res. 1985;10(6):535–549. [PubMed] [Google Scholar]
- Spiegel A. M., Shenker A., Weinstein L. S. Receptor-effector coupling by G proteins: implications for normal and abnormal signal transduction. Endocr Rev. 1992 Aug;13(3):536–565. doi: 10.1210/edrv-13-3-536. [DOI] [PubMed] [Google Scholar]
- Sutkowski E. M., Tang W. J., Broome C. W., Robbins J. D., Seamon K. B. Regulation of forskolin interactions with type I, II, V, and VI adenylyl cyclases by Gs alpha. Biochemistry. 1994 Nov 1;33(43):12852–12859. doi: 10.1021/bi00209a017. [DOI] [PubMed] [Google Scholar]
- Taussig R., Tang W. J., Hepler J. R., Gilman A. G. Distinct patterns of bidirectional regulation of mammalian adenylyl cyclases. J Biol Chem. 1994 Feb 25;269(8):6093–6100. [PubMed] [Google Scholar]
- Watson P. A., Krupinski J., Kempinski A. M., Frankenfield C. D. Molecular cloning and characterization of the type VII isoform of mammalian adenylyl cyclase expressed widely in mouse tissues and in S49 mouse lymphoma cells. J Biol Chem. 1994 Nov 18;269(46):28893–28898. [PubMed] [Google Scholar]
- Whaley B. S., Yuan N., Birnbaumer L., Clark R. B., Barber R. Differential expression of the beta-adrenergic receptor modifies agonist stimulation of adenylyl cyclase: a quantitative evaluation. Mol Pharmacol. 1994 Mar;45(3):481–489. [PubMed] [Google Scholar]
- Yee R., Liebman P. A. Light-activated phosphodiesterase of the rod outer segment. Kinetics and parameters of activation and deactivation. J Biol Chem. 1978 Dec 25;253(24):8902–8909. [PubMed] [Google Scholar]